Hepatitis Testing - CAM 127
Description
Infectious hepatitis is an inflammation of the liver caused by the hepatitis viruses. Hepatitis C is a blood-borne virus that can be spread via sharing needles or other equipment to inject drugs as well as in inadequate infection control in healthcare settings (CDC, 2018). Hepatitis C causes liver disease and inflammation. A chronic HCV infection can lead to hepatic damage, including cirrhosis and hepatocellular carcinoma, and is the most common cause of liver transplantation in the United States (AASLD-IDSA, 2015).
Hepatitis B is spread by the “Percutaneous, mucosal, or nonintact skin exposure to infectious blood, semen, and other body fluids.” As the hepatitis B virus is concentrated most highly in blood, “percutaneous exposure is an efficient mode of transmission,” though HBV can also be transmitted through birth to an infected mother and sexual contact with an infected person and less commonly through needle-sticks or other sharp instrument injuries, organ transplantation and dialysis, and interpersonal contact through sharing items, such as razors or toothbrushes or contact with open sores of an infected person. Similar to HCV infection, 15% to 25% of people with chronic HBV infection develop chronic liver disease (CDC, 2020a).
The general route of transmission for the hepatitis A virus is through the fecal-oral route by close person-to-person contact with an infected person, sexual contact with an infected person, or the ingestion of contaminated food or water, with the bloodborne transmission of HAV being uncommon (CDC, 2020a). Though death is uncommon and most people with acute HAV infection recover with no lasting liver damage, HAV remains a worldwide public health issue and is endemic in many low- to middle-income countries (CDC, 2020a; Keles et al., 2021).
For HCV and HBV screening in pregnant individuals, please see CAM 119 Prenatal Screening (Nongenetic).
Regulatory Status
Food and Drug Administration (FDA)
Many labs have developed specific tests that they must validate and perform in house. These laboratory-developed tests (LDTs) are regulated by the Centers for Medicare & Medicaid Services (CMS) as high-complexity tests under the Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88). LDTs are not approved or cleared by the U.S. Food and Drug Administration; however, FDA clearance or approval is not currently required for clinical use.
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
Hepatitis B
- For all individuals 18 years of age and older, triple panel testing (hepatitis B surface antigen [HBsAg], hepatitis B surface antibody [anti-HBs], total antibody to hepatitis B core antigen [anti-HBc]) for Hepatitis B (HBV) infection once per lifetime is considered MEDICALLY NECESSARY.
- For asymptomatic, non-pregnant individuals, the following annual HBV infection screening is considered MEDICALLY NECESSARY:
- HBsAg and hepatitis B surface antibody (anti-HBs) for infants born from an HBsAg-positive individual.
- Triple panel testing (HBsAg, anti-HBs, anti-HBc) when one of the following high-risk situations is met:
- For individuals born in or who have recently traveled to geographic regions with a HBV prevalence 2% or higher (see Note 1).
- For U.S.-born individuals not vaccinated as infants whose parents were born in geographic regions with an HBV prevalence 8% or higher (see Note 1).
- For individuals with a history of incarceration.
- For individuals infected with HIV.
- For individuals with a history of sexually transmitted infections or multiple sex partners.
- For men who have sex with men.
- For household contacts, needle-sharing contacts, and sex partners of HBV-infected individuals.
- For injection-drug users.
- For individuals with an active hepatitis C virus infection or who have a history of hepatitis C virus infection.
- For individuals with elevated liver enzymes.
- For individuals who are on long-term hemodialysis treatment.
- For individuals with diabetes.
- For health care and public safety workers exposed to blood or body fluids.
- For individuals who test positive for anti-HBc, follow up IgM antibody to anti-HBc (IgM anti-HBc) testing to distinguish between an acute or chronic infection is considered MEDICALLY NECESSARY.
- For the confirmation of seroconversion after hepatitis B vaccination, anti-HBs testing is considered MEDICALLY NECESSARY.
- For individuals who test positive for HBV by initial antibody screening and who will undergo immunosuppressive drug therapy, HBV DNA testing is considered MEDICALLY NECESSARY.
Hepatitis C
- For all individuals 18 years of age and older, antibody testing for Hepatitis C (HCV) infection once per lifetime is considered MEDICALLY NECESSARY.
- For any individual with the following recognized conditions or exposures, one-time, post-exposure antibody testing for HCV infection is considered MEDICALLY NECESSARY:
- For individuals who have used illicit intranasal or injectable drugs.
- For individuals who have received clotting factor concentrates produced before 1987.
- For individuals with a history of hemodialysis.
- For individuals with evidence of liver disease (based on clinical presentation, persistently abnormal ALT levels, or abnormal liver function studies).
- For individuals infected with HIV.
- For individuals who received an organ transplant before July 1992.
- For individuals who received a blood transfusion or blood component before July 1992.
- For individuals notified that they received blood from a donor who later tested positive for an HCV infection.
- For individuals with a history of incarceration.
- For individuals who received a tattoo in an unregulated setting.
- For healthcare, emergency medical, and public safety workers after needle sticks, sharps, or mucosal exposures to HCV-positive blood.
- For children born from an HCV-positive individual.
- For current sexual partners of HCV-infected persons.
- Routine periodic antibody testing for HCV is considered MEDICALLY NECESSARY for individuals with any of the following ongoing risk factors (while risk factors persist):
- For individuals who currently inject drugs and share needles, syringes, or other drug preparation equipment.
- For individuals who are receiving ongoing hemodialysis.
- For individuals engaging in high-risk sexual behavior.
- Qualitative nucleic acid testing for HCV is considered MEDICALLY NECESSARY in any of the following situations:
- As a follow up for individuals who test positive for HCV by initial antibody screening (to differentiate between active infection and resolved infection).
- One time screening for perinatally exposed infants who are 2-6 months of age.
- Prior to the initiation of direct acting anti-viral (DAA) treatment, one time testing for HCV genotype to guide selection of the most appropriate antiviral regimen is considered MEDICALLY NECESSARY.
- Testing for HCV viral load with a quantitative nucleic acid test is considered MEDICALLY NECESSARY in any of the following situations:
- Prior to the initiation of DAA therapy.
- After four weeks of DAA therapy.
- At the end of treatment.
- Twelve, twenty-four, and forty-eight weeks after completion of treatment.
Hepatitis A
- For individuals with signs and symptoms of acute viral hepatitis and who have tested negative for HBV and HCV, testing for IgM anti-hepatitis A (HAV) or qualitative testing for HAV RNA is considered MEDICALLY NECESSARY.
- Quantitative nucleic acid testing for HAV viral load is considered NOT MEDICALLY NECESSARY.
Hepatitis D
- For individuals who have tested positive for HBV, testing for hepatitis D virus (HDV) antibody (anti-HDV) or qualitative testing for HDV RNA is considered MEDICALLY NECESSARY.
- Quantitative nucleic acid testing for HDV viral load is considered NOT MEDICALLY NECESSARY.
NOTES:
Note 1: The CDC defines HBsAg prevalence by geographic region: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/hepatitis-b.
Table of Terminology
| Term |
Definition |
| AASLD |
American Association for the Study of Liver Diseases |
| Ab |
Antibody |
| AFP |
Alpha fetoprotein |
| Ag |
Antigen |
| AGA |
American Gastroenterological Association |
| ALT |
Alanine aminotransferase |
| anti-HBc |
Total antibody to hepatitis B core antigen |
| anti-HBs |
Hepatitis B surface antibody |
| aPTT |
Activate partial thromboplastin time |
| AST |
Aspartate aminotransferase |
| CBC |
Complete blood count |
| CDC |
Centers for Disease Control and Prevention |
| CHC |
Chronic hepatitis C |
| CIA |
Chemiluminescence immunoassays |
| CLIA ’88 |
Clinical Laboratory Improvement Amendments of 1988 |
| CMS |
Centers for Medicare & Medicaid Services |
| DAA |
Direct acting anti-viral |
| DBS |
Dried blood spot |
| EASL |
European Association for the Study of the Liver |
| EIA |
Enzyme immunoassays |
| ELISA |
Enzyme-linked immunosorbent assay |
| FBC |
Full blood count |
| FDA |
Food and Drug Administration |
| HAV |
Hepatitis A virus |
| HBsAg |
Hepatitis B surface antigen |
| HBV |
Hepatitis B virus |
| HCR |
Hepatitis C Screening |
| HCV |
Hepatitis C virus |
| HIV |
Human immunodeficiency virus |
| ICP |
Intrahepatic cholestasis of pregnancy |
| IDSA |
Infectious Disease Society of America |
| IgM |
Immunoglobulin M |
| IgM anti-HBc |
IgM antibody to anti-HBc |
| IHS |
Indian Health Services |
| INR |
International normalized ratio |
| LDH |
Lactic acid dehydrogenase |
| LDTs |
Laboratory-developed tests |
| MTCT |
Mother-to-child transmission |
| NAAT |
Nucleic acid amplification test |
| NAT |
Nucleic acid test |
| NCD |
National coverage determinations |
| PALF |
Pediatric acute liver failure |
| PCR |
Polymerase chain reaction |
| POC |
Point of care |
| PrEP |
Pre-exposure prophylaxis |
| PT |
Prothrombin time |
| PWID |
People who inject drugs |
| Rdts |
Rapid diagnostic tests |
| RNA |
Ribonucleic acid |
| SVR |
Sustained virologic response |
| T2DM |
Type 2 diabetes mellitus |
| TB |
Tuberculosis |
| USPSTF |
United States Preventive Services Task Force |
| WHO |
World Health Organization |
Rationale
Hepatitis C
The Centers for Disease Control and Prevention estimate that 2.4 million people in the United States have chronic hepatitis C (CDC, 2020b). Prevalence of the infection is highest in individuals born between 1945 and 1965. This rate is approximately six times higher than that seen in other adult age groups, and the CDC estimated approximately 50,300 new infections occurring each year (CDC, 2018). Hepatitis C virus (HCV) infection is the most common reason for liver transplantation in adults in the U.S. and may lead to hepatocellular carcinoma (Chopra, 2024).
It is estimated that 20% of people with HCV infection will develop cirrhosis, and nearly five percent will die from liver disease resulting from the HCV infection. The number of deaths from hepatitis is increasing and is projected to continue to increase for several more decades unless treatment is scaled up considerably (Razavi et al., 2014). Although HCV infection is common, it is estimated that 50-75% of individuals who are infected are unaware of their infection as symptoms are absent or nonspecific until much later, and therefore do not receive the care and treatment that can mitigate progression to severe liver disease and possibly death (Hagan et al., 2006; Rein et al., 2012).
Hepatitis C virus is spread through exposure to blood of infected individuals. Such exposure includes injection drug use, blood transfusions (prior to 1992), and to a lesser extent, high-risk sexual behaviors. Additionally, being born to an HCV-infected mother, hemodialysis, intranasal drug use, tattoos, incarceration, needle sticks, and invasive procedures (prior to implementation of universal precautions) are also associated with increased risk of HCV infection. Some countries are experiencing a recent resurgence of HCV infection among young intravenous drug users and HIV-infected homosexual men (CDC, 2015; Wandeler et al., 2015).
Hepatitis C virus is a small, positive-stranded RNA-enveloped virus with a highly variable genome (Simmonds, 2001). Assessment of the HCV genotype is crucial for management of the HCV infection. There are currently six major genotypes of HCV, and major treatment decisions (regimen, dosing, duration) vary from genotype to genotype (Chopra & Arora, 2024a). Some regimens for one genotype (such as ledipasvir-sofosbuvir [“Harvoni”] for genotype one) may not be effective for another (in this case, Harvoni may be used for genotypes one, four, five, and six but not two or three) (Lexidrug, 2024; Muir & Graham, 2024).
Hepatitis C virus is frequently asymptomatic, necessitating the need of strong screening procedures. As many as 50% of HCV-infected individuals are unaware of their diagnosis, and risk factors such as drug use or blood transfusions may increase risk of acquiring an HCV infection. Several expert groups, such as the CDC, have delineated screening recommendations in order to provide better care against the virus (Chopra & Arora, 2024b).
Hepatitis C can be diagnosed with either serologic antibody assays or molecular RNA tests. A serologic assay can detect an active infection and a resolved HCV infection, but cannot differentiate whether the infection is acute, chronic, or no longer present. Various serologic assays include enzyme immunoassays (EIA), chemiluminescence immunoassays (CIA), and point-of-care rapid immunoassays (Spach, 2020).
Molecular RNA tests detect Hepatitis C RNA, and the process includes nucleic acid test (NAT) or nucleic acid amplification test (NAAT). The NAT test becomes positive one to two weeks after initial infection and it has become the gold standard test for patients who have a positive EIA screening test. The NAT can detect whether a patient has a current active infection or a resolved infection (Spach, 2020).
Hepatitis B
The hepatitis B virus (HBV) is a double-stranded DNA virus belonging to the hepadnavirus family. The diagnosis of its acute infection is characterized by the detection of hepatitis B surface antigen (HBsAg) and immunoglobulin M (IgM) antibody to hepatitis B core antigen (anti-HBc), and chronic conditions develop in 90% of infants after acute infection at birth, 25%–50% of children newly infected at ages one to five years, and five percent of people newly infected as adults (CDC, 2020a; Lok, 2021).
Hepatitis B virus is transmitted from infected patients to those who are not immune (i.e., hepatitis B surface antibody [anti-HBs]-negative). Methods of transmission include mother-to-child (whether in utero, at birth, or after birth), breastfeeding, paternal transmission (i.e., close contact with infected blood or fluid of fathers), transfusion, sexual transmission, nosocomial infection, percutaneous inoculation, transplantation, and blood exposure via minor breaks in skin or mucous membranes (Teo & Lok, 2022).
In the United States, an estimated 862,000 people were living with chronic hepatitis B infection in 2016, with 21,600 new infections in 2018. Though most people with acute disease recover with no lasting liver damage, 15% to 25% of those with chronic disease develop chronic liver disease, including cirrhosis, liver failure, or liver cancer. It is believed that there are more than 250 million HBV carriers in the world, 600,000 of whom die annually from HBV-related liver diseases. As many as 60% of HBV-infected persons are unaware of their infection, and many remain asymptomatic until the presentation of cirrhosis or late-stage liver disease (CDC, 2020a; Krist et al., 2020; Lok, 2021).
The initial evaluation of chronic HBV infection should include a history and physical examination focusing on “risk factors for coinfection with hepatitis C virus (HCV), hepatitis delta virus (HDV), and/or HIV; use of alcohol; family history of HBV infection and hepatocellular carcinoma (HCC); and signs and symptoms of cirrhosis.” Furthermore, it should employ laboratory tests, such as “a complete blood count with platelets, liver chemistry tests (aspartate aminotransferase [AST], alanine aminotransferase [ALT], total bilirubin, alkaline phosphatase, albumin), international normalized ratio (INR), and tests for HBV replication (HBeAg, antibody to HBeAg [anti-HBe], HBV DNA”, and testing for hepatitis A virus (HAV) immunity with HAV immunoglobulin G (IgG) antibody in those who are not immune. Other considerations include evaluation for other causes of liver disease, screening for HIV infection, screening for hepatocellular carcinoma (HCC), screening for fibrosis, and, in rare cases, a liver biopsy (Lok, 2021).
Hepatitis A
Hepatitis A infection is caused by the hepatitis A virus, of which humans are the only known reservoir. The HAV virus is member of the genus Hepatovirus in the family Picornaviridae, and other previously used names for HAV infection include epidemic jaundice, acute catarrhal jaundice, and campaign jaundice (Lai & Chopra, 2024).
The hepatitis A virus is generally transmitted through the fecal-oral route, either via person-to-person contact (e.g., transmission within households, within residential institutions, within daycare centers, among military personnel, or sexually) or consumption of contaminated food or water (consumption of undercooked foods or foods infected by food handlers). Additional modes of transmission include blood transfusion and illicit drug use, and it should be noted that maternal-fetal transmission has not yet been described (Lai & Chopra, 2024).
Globally, approximately 1.4 million new cases of HAV infection occur each year — in the United States alone, an estimated 24,900 new infections were detected in 2018. Acute infection by HAV is usually a self-limited disease, with fulminant manifestations of hepatic failure occurring in fewer than one percent of cases. However, symptomatic illness due to HAV still presents itself in seventy percent of adults. Consequently, “diagnosis of acute HAV infection should be suspected in patients with abrupt onset of prodromal symptoms (nausea, anorexia, fever, malaise, or abdominal pain) and jaundice or elevated serum aminotransferase levels, particularly in the setting of known risk factors for hepatitis A transmission” through detection of serum IgM anti-HAV antibodies due to its persistence throughout the duration of the disease (CDC, 2020a; Lai & Chopra, 2024).
Proprietary Testing
Many point-of-care tests have been developed to diagnose hepatitis C efficiently. These point-of-care tests are particularly important for diagnoses in economically impoverished areas. Examples of these tests include OraQuick, TriDot and SDBioline. The OraQuick HCV test is an FDA approved point-of-care test which utilizes a fingerstick and a small whole blood sample to detect the virus. This test is reportedly more than 98% accurate and provides results in 20 minutes (OraSure, 2013). The fourth Generation HCV Tri-Dot is a rapid test which can detect all subtypes of HCV with 100% sensitivity and 98.9% specificity (JMitra&Co, 2015). This test uses human serum or plasma and can provide results in three minutes. Finally, the SDBioline HCV is an immunochromatographic rapid test that can identify HCV antibodies in human serum, plasma, or whole blood (Inc., 2023). This test uses a safe fingerstick procedure to obtain a sample.
Hepatitis panel tests have also been developed. For example, the VIDAS® Hepatitis panel by BioMérieux tests for hepatitis A, B, C, and E in less than two hours. This panel includes 11 automated assays and is a rapid, reliable and simple testing method. (BioMérieux, 2022). Legacy Health’s Hepatitis Chronic Panel detects Hepatitis B and C within 24-48 hours through a CIA method (Health, 2023).
A hepatitis C vaccine is currently not available, although many vaccines are under development; barriers to the development of such a vaccine include virus diversity, a lack of knowledge of the immune responses when an infection occurs, and limited models for the testing of new vaccines (Ansaldi et al., 2014; Bailey et al., 2019). The World Health Organization hopes for a 90% reduction in new hepatitis C cases by the year 2030 (Bailey et al., 2019).
Management of HCV infection typically involves monitoring the effect of treatment. The goal of treatment is to achieve a “sustained virologic response” (SVR), which is defined as “an undetectable RNA level 12 weeks following the completion of therapy” (Chopra & Pockros, 2024). This measure is a proxy for elimination of HCV RNA. The assessment schedule may vary regimen to regimen, but the viral load is generally evaluated every few weeks (Chopra & Pockros, 2024).
In 2023, the Biden-Harris administration called on Congress to embrace its proposed five-year program to eliminate hepatitis C in the United States. This five-year program was developed through extensive consultations with key stakeholders from both within and outside of the government, including patient groups, physician groups, and federal agencies. The program aims to significantly expand screening, testing, treatment, prevention, and monitoring of hepatitis C infections in the United States and specifically focuses on populations that are at the greatest risk for infection. One main priority in this national program is to accelerate the availability of point-of-care (POC) diagnostic tests. Hepatitis C RNA diagnostic POC tests are currently available outside of the United States, allowing for a test-and-treat approach in a single visit. “The administration proposal will support the Independent Technology Assessment Program, a collaboration between the Food and Drug Administration and the National Institutes of Health, the speed up clearance or approvals for such tests, just as was done by this same group for COID-19 POC tests.” It is believed that the availability of such POC tests will be game-changing for hepatitis C single-visit programs, particularly in “high-impact settings such as community health centers, substance use disorder treatment clinics, correctional facilities, emergency departments, and mobile vans” (Fleurence & Collins, 2023).
Clinical Utility and Validity
In order to determine the link between hepatitis A infection and its rare complication of acute liver failure in children in Somalia, a retrospective study was conducted on children aged 0 to 18 who were admitted to the pediatric outpatient clinic and pediatric emergency departments of the Somalia Mogadishu-Turkey Training and Research Hospital, Somali, from June 2019 and December 2019, and who were tested for HAV and had complete study data available (Keles et al., 2021). The authors found that of the 219 hepatitis A cases analyzed, 25 (11%) were diagnosed with pediatric acute liver failure (PALF) while the remaining 194 were not. It was found that children with PALF had “significantly had more prolonged PT and aPPT, and higher INR values in coagulation assays; and had higher levels of albumin in biochemical tests than the group without liver failure (for all, p ≤ 0.05)”, though no other significant differences were found based on the other laboratory parameters tested. Moreover, “Hepatic encephalopathy was observed in individuals with hepatitis A disease (12/219; 15.4%), in which PALF positive group (5/25;40%) was significantly higher compared to the non-PALF group (7/194; 4%) (p = < 0,001). The length of stay in the hospital or intensive care unit was significantly higher in children with acute liver failure (p = 0.001)”. As such, Keles et al. (2021) astutely notes that though “death rates of Hepatitis A infection seem to be low”, HAV infection may potentially “require long-term hospitalization of patients due to the complication of acute liver failure, which causes loss of workforce, constitutes a socio-economic burden on individuals and healthcare systems, and leads to mortality in settings where referral pediatric liver transplantation centers are not available”.
Spenatto et al. (2013) screened 6194 asymptomatic patients who were requesting an STI screening for hepatitis B infection. The authors found that only “male gender, lack of employment, and birth, in medium or high endemic country, were independently associated with HBsAg positivity in multivariate analysis”, and neither sexual behavior nor vaccination status are needed to target high-risk populations (Spenatto et al., 2013).
Su et al. (2022) evaluated the cost-effectiveness of implementing universal HBV screening in China to identify optimal screening strategies. By using a Markov cohort model, the researchers "simulated universal screening scenarios in 15 adult age groups between 18 and 70 years, with different years of screening implementation (2021, 2026, and 2031) and compared to the status quo (i.e., no universal screening)”, investigating a total of 180 different scenarios. Their work found suggested that “with a willingness-to-pay level of three times the Chinese gross domestic product (GDP) per capita (US$30 828), all universal screening scenarios in 2021 were cost-effective compared with the status quo”, with the “serum HBsAg/HBsAb/HBeAg/HBeAb/HBcAb (five-test) screening strategy in people aged 18-70 years was the most cost-effective strategy in 2021” and “the two-test strategy for people aged 18-70 years became more cost-effective at lower willingness-to-pay levels.” Most importantly, they claimed that the “five-test strategy could prevent 3·46 million liver-related deaths in China over the lifetime of the cohort” and that delaying strategic intervention will reduce overall cost-effectiveness (Su et al., 2022).
Messina et al. (2015) performed a meta-analysis on the prevalence of HCV genotypes worldwide. The authors evaluated 1217 studies encompassing approximately 90% of the global population. They calculated genotype one to comprise 83.4 million cases (46.2% of all HCV cases), genotype three to comprise 54.3 million cases (30.1%), and genotypes two, four, and six to comprise a combined 22.8% cases. Genotype five comprised less than one percent of HCV cases. The diversity of genotypes also varied; the highest diversity is observed in China and South-East Asia, while in some countries, such as Egypt and Mongolia, almost all HCV infections are caused by a single genotype (Messina et al., 2015).
Inoue et al. (2017) described four HCV patients whose treatment failed. These four HCV patients had received a treatment regimen of daclatasvir plus asunaprevir, which is used for genotype 1b. However, these four patients were re-tested and found to have a different genotype; three patients had genotype two and the fourth patient had genotype 1a. The authors suggested that the daclatasvir plus asunaprevir regimen was ineffective for patients without genotype 1b (Inoue et al., 2017).
Moreno et al. (2016) performed a cost analysis of expanded HCV coverage. Two scenarios were simulated, one with expanded fibrosis coverage to stage two fibrosis, and the other to all fibrosis cases. Over a 20-year simulation, treatment costs increased, but private payers experienced overall savings of $10 billion to $14 billion after treatment costs. A positive “spillover” benefit of $400 million to Medicare was seen in the five-year model, and a benefit of seven billion dollars to Medicare was seen in the 20-year model (Moreno et al., 2016).
Linthicum et al. (2016) assessed the cost-effectiveness of expanding screening and treatment coverage over a 20-year horizon. The authors investigated three scenarios, each of which expanded coverage to a different stage of fibrosis. “Net social value” was the primary outcome evaluated, and it was calculated by the “value of benefits from improved quality-adjusted survival and reduced transmission minus screening, treatment, and medical costs.” Overall, the scenario with only fibrosis stage three and fibrosis stage four covered generated $0.68 billion in social value, but the scenario with all fibrosis patients (stages zero to four) treated produced $824 billion in social value. The authors also noted that the scenario with all fibrosis stages covered created net social value by year nine whereas the scenario with only stages three and four covered needed all 20 years to break even (Linthicum et al., 2016).
Chen et al. (2019) completed a meta-analysis to research the relationship between type two diabetes mellitus development and patients with a HCV infection. Studies were included from 2010 to 2019. Five types of HCV individuals were incorporated in this study including those who were “non-HCV controls, HCV-cleared patients, chronic HCV patients without cirrhosis, patients with HCV cirrhosis and patients with decompensated HCV cirrhosis” (Chen et al., 2019). HCV infection was found to be a significant risk factor for type two diabetes mellitus development. Further, “HCV clearance spontaneously or through clinical treatment may immediately reduce the risk of the onset and development of T2DM [type 2 diabetes mellitus]” (Chen et al., 2019).
Saeed et al. (2020) completed a systematic review and meta-analysis of health utilities for patients diagnosed with a chronic hepatitis C infection. Health utility can be defined as a measure of health-related quality or general health status. A total of 51 studies comprised of 15,053 patients were included in this study. The researchers have found that “Patients receiving interferon-based treatment had lower utilities than those on interferon-free treatment (0.647 vs 0.733). Patients who achieved sustained virologic response (0.786) had higher utilities than those with mild to moderate CHC [chronic hepatitis C]. Utilities were substantially higher for patients in experimental studies compared to observational studies” (Saeed et al., 2020). Overall, these results show that chronic hepatitis C infections are significantly harming global health status based on the measurements provided by health utility instruments.
Vetter et al. (2022) conducted a retrospective study to assess the performance of rapid diagnostic tests (RDTs) for Hepatitis C virus (HCV) infection. Thirteen RDTs were studied including the Standard Q HCV Ab by SD Biosensor, HCV Hepatitis Virus Antibody Test by Antron Laboratories, HCV-Ab Rapid Test by Beijing Wantal Biological Pharmacy Enterprise, Rapid Anti-HCV Test by InTec, First Response HCV Card Test by Premier Medical Corporation, Signal HCV Version 3.0 by Arkray Healthcare, TRI DOT HCV by J. Mitra & Co, Modified HCV-only Ab Test by Biosynex SA, SD Bioline HCV by Abbott Diagnostics, OraQuick Hepatitis C virus by OraSure, Prototype HCV Ab Test by BioLytical Laboratories, Prototype DPP HCV by Chembio Diagnostic Systems, and Prototype Care Start HCV by Access Bio. A total of 1,710 samples were evaluated in which 648 samples were HCV positive and 264 samples were also HIV positive. In the samples from HIV negative patients, most RDTs showed high sensitivity of > 98% and specificity of >99%. In HIV positive patients, sensitivity was lower with only one RDT reaching >95%. However, specificity was higher, with only four RDTs showing a specificity of <97%. The authors concluded that these tests are compliant with the World Health Organization (WHO) guidance which recommends an HCV RDT to have a sensitivity of >98% and specificity >97%. However, in HIV positive patients, the specificity remained high, but none of the tests met the WHO sensitivity criteria. The authors conclude that "these findings serve as a valuable baseline to investigate RDT performance in prospectively collected whole blood samples in the intended use settings” (Vetter et al., 2022).
In a prospective study, Chevaliez et al. (2020) evaluated the use of molecular point of care (POC) testing and dried blood spot (DBS) for HCV screening in people who inject drugs (PWID). A total of 89 HCV-seropositive PWID were further assessed with a liver assessment, blood tests, POC HCV RNA testing, and fingerstick DBS sampling. A total of 77 patients had paired fingerstick capillary whole blood for POC HCV RNA testing and fingerstick sampling with interpretable results, while the other 12 samples had no valid result due to low sample volume. The POC HCV RNA test detected 30 HCV-seropositive PWID and DBS sampling detected 27 HCV-seropositive PWID. The rate of invalid results using the POC test was below 10%, so it may be performed by staff without extensive clinical training in decentralizing testing location. This study also showed high concordance for detection of active HCV infection from DBS compared to the POC test. The authors conclude that the use of POC diagnostic testing and DBS sampling should be recommended as a one-step screening strategy to increase diagnosis, increase treatment, and reduce the number of visits.
In an Australian observational study, Catlett et al. (2021) evaluated the Aptima HCV Quant Dx Assay to see how well it could detect HCV RNA from fingerstick capillary dried blood spot (DBS) and venipuncture-collected samples. DBS collection would benefit marginalized populations in areas that may not have access to phlebotomy services or who may have difficult venous access. DBS has also been shown to “enhance HCV testing and linkage to care,” be easy for transport and storage, and can be used for other purposes like HCV sequencing and testing for HIV or hepatitis B simultaneously, which is useful in more resource-limited settings. From 164 participants, they found HCV RNA in 45 patients. The Aptima assay rendered a sensitivity and specificity of 100% from plasma, and a sensitivity of 95.6% and specificity of 94.1% from DBS. This demonstrated the comparable diagnostic performance of this assay when it comes to detecting active HCV infection from DBS samples and plasma samples, and hopefully the eventual use of other similar assays with similar performances.
Centers for Disease Control and Prevention (CDC)
Hepatitis C
The CDC recommends universal hepatitis C screening for
- “Hepatitis C screening at least once in a lifetime for all adults aged 18 years and older, except in settings where the prevalence of HCV infection (HCV RNA‑positivity) is less than 0.1%”
- “Hepatitis C screening for all pregnant individuals during each pregnancy, except in settings where the prevalence of HCV infection (HCV RNA‑positivity) is less than 0.1%” (CDC, 2023a).
Moreover, one-time hepatitis C testing regardless of age or setting prevalence among people with recognized conditions or exposures is recommended for the following groups:
- “People who currently or have previously injected drugs and shared needles, syringes, or other drug preparation equipment.
- People with human immunodeficiency virus (HIV).
- People with selected medical conditions, including
people who have ever received maintenance hemodialysis and persons with persistently abnormal alanine aminotransferase (ALT) levels.
- Prior recipients of transfusions or organ transplants, including:
- People who received clotting factor concentrates produced before 1987.
- People who received a transfusion of blood or blood components before July 1992.
- People who received an organ transplant before July 1992.
- People who were notified that they received blood from a donor who later tested positive for HCV infection.
- Health care, emergency medical, and public safety personnel after needle sticks, sharps, or mucosal exposures to HCV-positive blood.
- Infants born to people with known hepatitis C”.
It is also stated that “Routine periodic testing is recommended for people with ongoing risk factors (regardless of setting prevalence), including:
- People who currently inject drugs and share needles, syringes, or other drug preparation equipment
- People with selected medical conditions, including people who ever received maintenance hemodialysis.”
It is also recommended that “Clinicians should test anyone who requests a hepatitis C test, regardless of stated risk factors, because patients may be hesitant to share stigmatizing risks” (CDC, 2023a).
CDC screening and testing guidelines state that “Clinicians should initiate hepatitis C testing with an HCV antibody test with reflex to NAT for HCV RNA if the antibody test is positive/reactive.” Moreover, the CDC provides operational guidance for complete hepatitis C testing, noting that “It is important to reduce time to diagnosis, evaluation, and treatment initiation. CDC recommends that clinicians collect all samples needed to diagnose hepatitis C in a single visit and order HCV RNA testing automatically when the HCV antibody is reactive” and that “When the HCV antibody test is reactive, the laboratories should automatically perform NAT testing for HCV RNA detection. This automatic testing streamlines the process because it occurs without any additional action on the part of the patient or the clinician” (CDC, 2023a).
Furthermore, “HCV RNA testing is recommended for the diagnosis of current HCV infection among people who might have been exposed to HCV within the past 6 months, regardless of HCV antibody result.”
The CDC asserts that “Clinicians should use an FDA-approved HCV antibody test followed by a NAT for HCV RNA test when antibody is positive/reactive.” Such tests include
- HCV antibody test (anti-HCV) (e.g., enzyme immunoassay [EIA]).
- Nucleic acid test (NAT) to detect presence of HCV RNA (qualitative RNA test).
- NAT to detect levels of HCV RNA (quantitative RNA test) (CDC, 2023a).
The CDC notes that “A reactive HCV antibody test result indicates a history of past or current HCV infection. A detectable HCV RNA test result indicates current infection” and urge that “NAT for detection of HCV RNA should be used among people with suspected HCV exposure within the past 6 months.”
- For perinatally exposed infants, the CDC notes that “Clinicians should test all perinatally exposed infants for HCV RNA
- using a NAT at 2 – 6 months. Care for infants with detectable HCV RNA should be coordinated in consultation with a provider who has expertise in pediatric hepatitis C management.
Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted” (CDC, 2023a).
The CDC also notes that the initial HCV test should be “with an FDA-approved test for antibody to HCV.” A positive result for the HCV antibody indicates either a current infection or previous infection that has resolved. For those individuals, the CDC recommends testing by an FDA-approved HCV nucleic acid test (NAT) to differentiate between active infection and resolved infection. For the identification of chronic hepatitis C virus infection among persons born between 1945 and 1965, the CDC states that “Persons who test anti-HCV positive or have indeterminate antibody test results who are also positive by HCV NAT should be considered to have active HCV infection; these persons need referral for further medical evaluation and care.” Finally, the CDC also recommends that repeat testing should be considered for individuals with ongoing risk behaviors (CDC, 2012).
The CDC published guidance for healthcare personnel with potential exposure to HCV. CDC recommends testing the source patient and the healthcare personnel. When testing the source patient, baseline testing should be performed within 48 hours after exposure by testing for HCV RNA or HCV antibodies. All HCV RNA testing should be performed with a nucleic acid test. If the source patient was HCV RNA positive or if source patient testing was not performed, baseline testing for healthcare personnel should follow the same steps through nucleic acid testing three to six weeks post-exposure. A final HCV antibody test should be performed at four to six months post-exposure to ensure a negative HCV RNA test result (CDC, 2020d).
No serologic marker for acute infection is available, but for chronic infections, CDC propounds the use of “Assay for anti-HCV” and “Qualitative and quantitative nucleic acid tests (NAT) to detect and quantify presence of virus (HCV RNA)” (CDC, 2020a).
Hepatitis B
The CDC offers guidance on how to make decisions on whether to test or screen for hepatitis B based on the demographic.
- For adults: “CDC recommends screening all adults aged 18 and older for hepatitis B at least once in their lifetime using a triple panel test
To ensure increased access to testing, anyone who requests HBV testing should receive it regardless of disclosure of risk. Many people might be reluctant to disclose stigmatizing risks.”
For infants: “CDC recommends testing all infants born to HBsAg-positive people for HBsAg and antibody to hepatitis B surface antigen (anti-HBs) seromarkers.”
For pregnant people: “CDC recommends HBV screening for HBsAg for all pregnant people during each pregnancy, preferably in the first trimester, regardless of vaccination status or history of testing. Pregnant people with a history of appropriately timed triple panel screening without subsequent risk for exposure to HBV (no new HBV exposures since triple panel screening) only need HBsAg screening.”
For people at increased risk: “CDC recommends testing susceptible people periodically, regardless of age, with ongoing risk for exposures while risk for exposures persists. This includes:
- People with a history of sexually transmitted infections or multiple sex partners.
- People with history of past or current HCV infection.
- People incarcerated or formerly incarcerated in a jail, prison, or other detention setting.
- Infants born to HBsAg-positive people.
- People born in regions with HBV infection prevalence of 2% or more.
- US-born people not vaccinated as infants whose parents were born in geographic regions with HBsAg prevalence of 8% or more.
- People who inject drugs or have a history of injection drug use.
- People with human immunodeficiency virus (HIV) infection.
- Men who have sex with men.
- Household contacts or former household contacts of people with known HBV infection.
- People who have shared needles with or engaged in sexual contact with people with known HBV infection.
- People on maintenance dialysis, including in-center or home hemodialysis and peritoneal dialysis.
- People with elevated liver enzymes” (CDC, 2024b).
The CDC also explains that “Susceptible people include those who have never been infected with HBV and either did not complete a HepB vaccine series per ACIP recommendations or who are known to be vaccine nonresponders.”
The CDC states that they now* recommend the use of the triple panel test, which includes testing for
- HBsAg
- Anti-HBs
- Total antibody to hepatitis B core antigen (total anti-HBc). This differs from prior guidance (hence the asterisk *), which recommended a single test of HBsAg.
It is noted that “Any periodic follow-up testing can use tests as appropriate based on the results of the triple panel” (CDC, 2024b).
The table below provides CDC recommendations for screening, testing and vaccination for children and adults based on population groups. Infants and Young Adolescents (CDC, 2023c):
| Population |
Recommendation |
|
| Screening and Testing |
Vaccination |
|
| Infants without known hepatitis B exposure |
None |
Routine vaccination of all infants with the hepatitis B vaccine series, with the first dose administered within 24 hours of birth |
| Infants born to hepatitis B surface antigen (HBsAg)-positive pregnant people |
Provide hepatitis B immune globulin (HBIG) and first dose of hepatitis B vaccine within 12 hours of birth, followed by completion of the vaccine series and postvaccination serologic testing See Hepatitis B Vaccination of Infants – Adolescents | CDC |
|
| Infants born to pregnant people for whom HBsAg testing results during pregnancy are not available but for whom other evidence suggests maternal HBV infection (e.g., HBV DNA, HBeAg-positive, or pregnant person known to be chronically infected with HBV |
For infants equal to or more than 2,000 grams, provide first dose of hepatitis B vaccine within 12 hours of birth, followed by completion of the vaccine series For infants with birthweight less than 2,000 grams, provide hepatitis B immune globulin (HBIG) and first dose of hepatitis B vaccine within 12 hours of birth, followed by completion of the vaccine series and postvaccination serologic testing |
|
| Adolescents under age 19 years who have not been vaccinated and with no known risk factors |
None |
Vaccinate |
Older Adolescents and Adults (CDC, 2023c):
| Population |
Recommendation |
|
| Screening and Testing |
Vaccination |
|
| Adults with no known risk factors for hepatitis B |
If never previously screened, test for HBsAg, anti-HBs, and total anti-HBc (triple panel) |
Vaccinate adults aged 18 – 59 years |
| People with risk factors, regardless of age, such as:
|
If never previously screened, test for HBsAg, anti-HBs, and total anti-HBc (triple panel)
If previously screened, but still unvaccinated, offer testing to people who have ongoing risk for exposure For additional screening considerations for patients on dialysis, see: Recommendations for Preventing Transmission of Infections Among Chronic Hemodialysis Patients (cdc.gov) |
Vaccinate For additional considerations for patients on dialysis, see Recommendations for Preventing Transmission of Infections Among Chronic Hemodialysis Patients (cdc.gov) |
| Other populations at risk:
|
If never previously screened, test for HBsAg, anti-HBs, and total anti-HBc (triple panel)
For additional screening considerations for patients on dialysis see: Recommendations for Preventing Transmission of Infections Among Chronic Hemodialysis Patients (cdc.gov) |
Vaccinate For additional vaccination considerations for healthcare personnel see: Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices | MMWR (cdc.gov) |
Serologic tests for chronic hepatitis B infections should include three HBV seromarkers: HBsAg, anti-HBs, and Total anti-HBc, while testing for acute infection should include HBsAg and IgM anti-HBc. The CDC provides the following chart on interpreting serologic testing results:
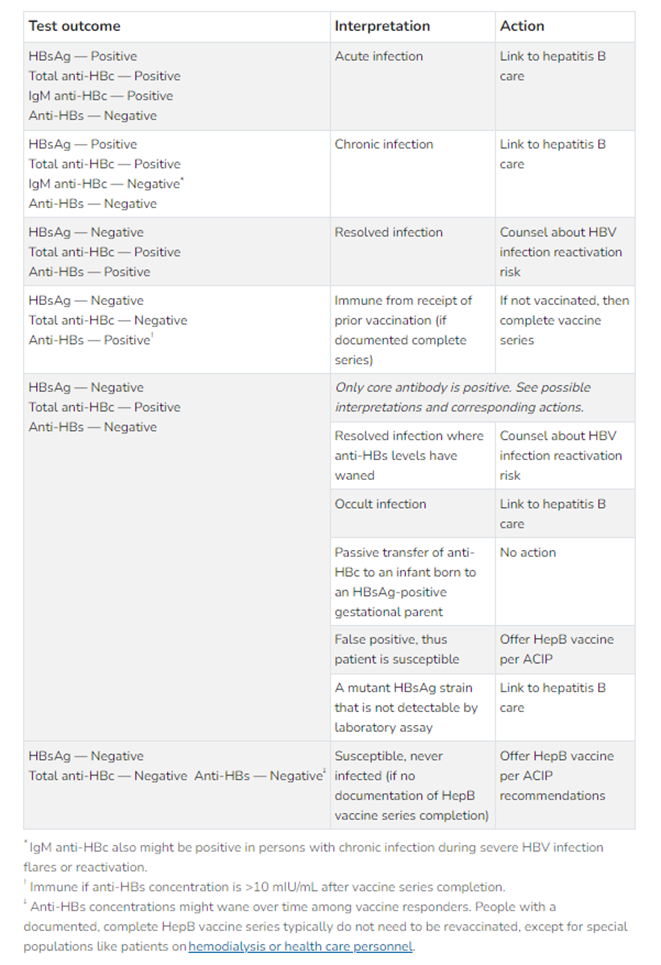
Figure 1: Interpreting HBV serologic test results (CDC, 2024b)
For health care providers and viral hepatitis, the CDC makes the following recommendation: “Health care providers should be vaccinated against hepatitis B and tested for hepatitis C after a potential exposure. ... For continued protection, CDC and the Advisory Committee on Immunization Practices (ACIP) recommend that health-care providers and public-safety workers with reasonably anticipated risk for exposures to blood or infectious body fluids receive the complete hepatitis B vaccine series and have their immunity documented through postvaccination testing” (CDC, 2023b).
Hepatitis A
Hepatitis A does not present as a chronic infection; as such, the CDC offers no testing recommendations (CDC, 2020a). The CDC lists the following clinical features when infected with HAV:
- Abdominal pain, nausea, and/or vomiting
- Dark urine or clay-colored stools
- Diarrhea
- Fatigue
- Fever
- Jaundice
- Joint pain
- Loss of appetite (CDC, 2024a).
However, it should be noted that “In children younger than 6, 70% of infections are asymptomatic. When symptoms do present, young children typically do not have jaundice, whereas most older children and adults with HAV infection have jaundice” (CDC, 2024a).
The CDC cautions that “You will not be able to differentiate hepatitis A virus from other types of viral hepatitis using clinical or epidemiological features alone. Clinicians should conduct test(s) to make an accurate diagnosis.” As such, they assert that “The following are laboratory markers that, if present, indicate an acute HAV infection”
- Immunoglobulin M antibodies to HAV (IgM anti-HAV) in serum, or
- HAV RNA in serum or stool (CDC, 2024a).
The CDC notes that the presence of immunoglobulin G antibodies to HAV (IgG anti-HAV) indicates either immunity from prior infection or vaccination.
Not all tests are created equal, however; it should be mentioned that “Serologic tests for IgG anti-HAV and total anti-HAV (IgM and IgG anti-HAV combined) are not helpful in diagnosing acute illness. You should only test patients for IgM anti-HAV if they are symptomatic, and you suspect HAV infection. Alanine aminotransferase (ALT) and total bilirubin tests can aid in diagnosis” (CDC, 2024a).
Hepatitis D
According to the CDC, “HDV is known as a ‘satellite virus,’ because it can only infect people who are also infected by the hepatitis B virus (HBV). HDV infection can be acute or lead to chronic, long-term illness. The infection can be acquired either simultaneously with HBV as a coinfection or as a superinfection in people who are already chronically infected with HBV” (CDC, 2020c). Hepatitis D infections are not clinically distinguishable from other types of acute viral hepatitis and thus “diagnosis can be confirmed only by testing for the presence of antibodies against HDV and/or HDV RNA. HDV infection should be considered in any person with a positive hepatitis B surface antigen (HBsAg) who has severe symptoms of hepatitis or acute exacerbations” (CDC, 2020c).
United States Preventive Services Task Force (USPSTF)
The USPSTF recommends hepatitis C virus screening in adults aged 18 to 79 years (B recommendation) with anti-HCV antibody testing followed by confirmatory PCR testing (Owens et al., 2020).
The United States Preventive Services Task Force (USPSTF) recommends screening for hepatitis B virus (HBV) infection in adolescents and adults at increased risk for infection. This applies to all asymptomatic, nonpregnant adolescents and adults at increased risk for HBV infection, including those who were vaccinated before being screened for HBV infection. The USPSTF defines some increased-risk groups as “Persons born in the US with parents from regions with higher prevalence are also at increased risk of HBV infection during birth or early childhood, particularly if they do not receive appropriate passive and active immunoprophylaxis (and antiviral therapy for pregnant individuals with a high viral load)” and also “persons who have injected drugs in the past or currently; men who have sex with men; persons with HIV; and sex partners, needle sharing contacts, and household contacts of persons known to be HBsAg positive” (Krist et al., 2020).
USPSTF recommends the following in relation to screening tests for HBV: “Screening for hepatitis B should be performed with HBsAg tests approved by the US Food and Drug Administration, followed by a confirmatory test for initially reactive results. A positive HBsAg result indicates chronic or acute infection. Serologic panels performed concurrently with or after HBsAg screening allow for diagnosis and to determine further management” (Krist et al., 2020).
American Association for the Study of Liver Diseases (AASLD) and the Infectious Disease Society of America (IDSA)
AASLD-IDSA guidelines recommend one-time HCV testing in the following situations:
- “One-time, routine, opt out HCV testing is recommended for all individuals aged 18 years and older. Rating: I, B
- One-time HCV testing should be performed for all persons less than 18 years old with activities, exposures, or conditions or circumstances associated with an increased risk of HCV infection (see below). Rating: I, B
- Prenatal HCV testing as part of routine prenatal care is recommended with each pregnancy. Rating: I, B
- Periodic repeat HCV testing should be offered to all persons with activities, exposures, or conditions or circumstances associated with an increased risk of HCV exposure (see below). Rating: IIa, C
- Annual HCV testing is recommended for all persons who inject drugs, for HIV-infected men who have unprotected sex with men, and men who have sex with men taking pre-exposure prophylaxis (PrEP). Rating: IIa, C
Risk Activities
- Injection-drug use (current or ever, including those who injected once)
- Intranasal illicit drug use
- Use of glass crack pipes
- Male engagement in sex with men
- Engagement in chem sex (defined as the intentional combining of sex with the use of particular nonprescription drugs in order to facilitate or enhance the sexual encounter
Risk exposures
- Persons on long-term hemodialysis (ever)
- Persons with percutaneous/parenteral exposures in an unregulated setting
- Healthcare, emergency medical, and public safety workers after needlestick, sharps, or mucosal exposures to HCV-infected blood
- Children born to HCV-infected [individuals]
- Recipients of a prior transfusion or organ transplant, including persons who:
- Were notified that they received blood from a donor who later tested positive for HCV
- Received a transfusion of blood or blood components, or underwent an organ transplant before July 1992
- Received clotting factor concentrates produced before 1987.
- Persons who were ever incarcerated
Other considerations and circumstances
- HIV infection
- Sexually active persons about to start pre-exposure prophylaxis (PreP) for HIV
- Chronic liver disease and/or chronic hepatitis, including unexplained elevated alanine aminotransferase (ALT) levels.
- Solid organ donors (living and deceased) and solid organ transplant recipients” (AASLD-IDSA, 2022a)
Recommendations for Initial HCV Testing and Follow-up
- “HCV-antibody testing with reflex HCV RNA polymerase chain reaction (PCR) is recommended for initial HCV testing to establish the presence of active infection (as opposed to spontaneous or treatment-induced viral clearance). Rating: Class I, Level A
- Among persons with a negative HCV-antibody test who were exposed to HCV within the prior six months, HCV-RNA or follow-up HCV-antibody testing six months or longer after exposure is recommended. HCV-RNA testing can also be considered for immunocompromised persons. Rating: Class I, Level C
- Among persons at risk of reinfection after previous spontaneous or treatment-related viral clearance, initial HCV-RNA testing is recommended because a positive HCV-antibody test is expected. Rating: Class I, Level C
- Persons found to have a positive HCV-antibody test and negative results for HCV RNA by PCR should be informed that they do not have evidence of current (active) HCV infection but are not protected from reinfection. Rating: Class I, Level A
- Quantitative HCV-RNA testing is recommended prior to the initiation of antiviral therapy to document the baseline level of viremia (i.e., baseline viral load).
Rating: Class I, Level A - HCV genotype testing may be considered for those in whom it may alter treatment recommendations. Rating: Class I, Level A” (AASLD-IDSA, 2022a; Bhattacharya et al., 2023)
For diagnosing and monitoring acute HCV infection, AASLD-IDSA issued the following recommendation:
- “HCV antibody and HCV RNA testing are recommended when acute HCV infection is suspected due to exposure, clinical presentation, or elevated aminotransferase levels.” (Rating: Class I, Level C)
- “After the initial diagnosis of acute HCV with viremia (defined as quantifiable RNA), HCV treatment should be initiated without awaiting spontaneous resolution.”(Rating: Class I, Level B) (AASLD-IDSA, 2022b)
For monitoring patients who are starting hepatitis C treatment, are on treatment, or have completed therapy, AASLD-IDSA issued the following recommendations:
- “The following laboratory tests are recommended within six months prior to starting DAA (direct-acting antiviral) therapy:
- Complete blood count (CBC)
- International normalized ratio (INR)
- Hepatic function panel (i.e., serum albumin, total and direct bilirubin, alanine aminotransferase [ALT], aspartate aminotransferase [AST], and alkaline phosphatase levels)
- Estimated glomerular filtration rate (eGFR)
- The following laboratory tests are recommended any time prior to starting DAA therapy:
- Quantitative HCV RNA (HCV viral load)
- If a nonpangenotypic DAA will be prescribed, then test for HCV genotype and subtype” (Rating: Class I, Level C)
- “Quantitative HCV viral load testing is recommended 12 or more weeks after completion of therapy to document sustained virologic response (SVR), which is consistent with cure of chronic infection” (Rating: Class I, Level B) (AASLD-IDSA, 2023b)
Recommendations for Posttreatment Follow-Up for Patients in Whom Treatment Failed
- “Disease progression assessment every six to 12 months with a hepatic function panel, complete blood count (CBC), and international normalized ratio (INR) is recommended if patients are not retreated or fail a second or third DAA treatment course. (Rating: Class I, Level C)
- Surveillance for hepatocellular carcinoma with liver ultrasound examination, with or without alpha fetoprotein (AFP), every six months is recommended for patients with cirrhosis in accordance with the AASLD guidance on the diagnosis, staging, and management of hepatocellular carcinoma. Rating: Low, Conditional” (AASLD-IDSA, 2023b).
Recommendations for Monitoring HCV-Infected [Persons] During Pregnancy
- “As part of prenatal care, all pregnant [individuals] should be tested for HCV infection with each pregnancy, ideally at the initial visit. (Rating: I, B)
- HCV RNA and routine liver function tests are recommended at initiation of prenatal care for HCV-antibody–positive pregnant persons to assess the risk of mother-to-child transmission (MTCT) and severity of liver disease. (Rating: I, B)
- All pregnant individuals with HCV infection should receive prenatal and intrapartum care that is appropriate for their individual obstetric risk(s) as there is no currently known intervention to reduce MTCT. (Rating: I, B)
- In HCV-infected pregnant individuals with pruritus or jaundice, there should be a high index of suspicion for intrahepatic cholestasis of pregnancy (ICP) with subsequent assessment of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum bile acids. (Rating: I, B)
- HCV-infected individuals with cirrhosis should be counseled about the increased risk of adverse maternal and perinatal outcomes. Antenatal and perinatal care should be coordinated with a maternal-fetal medicine (i.e., high-risk pregnancy) obstetrician (Rating: I, B)” (AASLD-IDSA, 2023a).
Assessment of Liver Disease Severity
A section focused on determining the severity of liver diseases associated with an HCV infection is also included as part of the background of these AASLD-IDSA guidelines. The authors state the following:
“The severity of liver disease associated with chronic HCV infection is a key factor in determining the initial and follow-up evaluation of patients. Noninvasive tests using serum biomarkers, elastography, or liver imaging allow for accurate diagnosis of cirrhosis in most individuals (see pretreatment workup in When and in Whom to Initiate HCV Therapy). Liver biopsy is rarely required but may be considered if other causes of liver disease are suspected.
Noninvasive methods frequently used to estimate liver disease severity include:
- Liver-directed physical exam (normal in most patients)
- Routine blood tests (eg, ALT, AST, albumin, bilirubin, international normalized ratio [INR], and CBC with platelet count)
- Serum fibrosis marker panels
- Transient elastography
- Liver imaging (eg, ultrasound or CT scan)” (AASLD-IDSA, 2022a)
Testing of Perinatally Exposed Children and Siblings of Children with HCV Infection• All children born to individuals with acute or chronic hepatitis C should be tested for HCV infection.
- Antibody-based testing is recommended at or after 18 months of age. (I, A)
- Testing with an HCV-RNA assay can be considered in the first year of life, but the optimal timing of such testing is unknown. (IIa, C)
- Testing with an HCV-RNA assay can be considered as early as two months of age. (IIa, B)
- Repetitive HCV-RNA testing prior to 18 months of age is not recommended. (III, A)
- Children who are anti-HCV-positive after 18 months of age should be tested with an HCV RNA assay after age three to confirm chronic hepatitis C infection. (I, A)
- The siblings of children with vertically acquired chronic hepatitis C should be tested for HCV infection, if born from the same mother (I, C) (Ghany & Morgan, 2020)
Testing recommendations relating to the monitoring and medical management of children include
- “Routine liver biochemistries at initial diagnosis and at least annually thereafter are recommended to assess for HCV disease progression. (I, C)”
- “Disease severity assessment by routine laboratory testing and physical examination, as well as use of evolving noninvasive modalities (i.e., transient elastography, imaging, or serum fibrosis markers) is recommended for all children with chronic hepatitis C. (I, B)” (Ghany & Morgan, 2020).
American Association for the Study of Liver Diseases (AASLD)
Hepatitis B
The guidance statements surrounding screening for hepatitis B infection is (shown in more detail following declare that
- Screening should be performed using both HBsAg and anti-HBs.
- Screening is recommended in all persons born in countries with a HBsAg seroprevalence of ≥ 2%, U.S.-born persons not vaccinated as infants whose parents were born in regions with high HBV endemicity (≥8%), pregnant individuals, persons needing immunosuppressive therapy, and the at-risk groups listed in Table 3.
- Anti-HBs-negative screened persons should be vaccinated.
- Screening for anti-HBc to determine prior expo-sure is not routinely recommended but is an important test in patients who have HIV infection, who are about to undergo HCV or anti-cancer and other immunosuppressive therapies or renal dialysis, and in donated blood (or, if feasible, organs) (Terrault et al., 2018).
AASLD recommends that the following groups are at high risk for HBV infection and should be screened and immunized if seronegative (Terrault et al., 2018):

AASLD proposes the use of various screening methods for the diagnose of hepatitis B infection: “HBsAg and antibody to hepatitis B surface antigen (anti-HBs) should be used for screening (Table 4). Alternatively, antibody to hepatitis B core antigen (anti-HBc) can be utilized for screening as long as those who test positive are further tested for both HBsAg and anti-HBs to differentiate current infection from previous HBV exposure. HBV vaccination does not lead to anti-HBc positivity.” The interpretations and follow-up steps of the screening results are summarized in their table (Terrault et al., 2018):
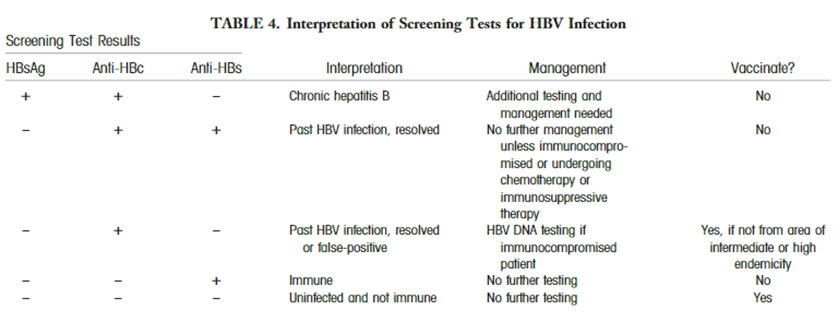
Hepatitis C
AASLD recommends not repeating hepatitis C viral load testing in patients with a previous positive (HCV) test, stating that “repeat HCV antibody testing adds cost but no clinical benefit.” They recommend “Instead, order hepatitis C viral load testing for assessment of active versus resolved infection.” This recommendation is also sponsored by the American Society for Clinical Pathology (AASLD, 2023).
World Health Organization (WHO)
Hepatitis C
Recommendations on screening for HCV infection (WHO, 2017, 2018):
| Testing approach |
Recommendations |
| Focused testing |
In all settings (and regardless of whether delivered through facility- or community-based testing), it is recommended that serological testing for HCV antibody (anti-HCV) be offered with linkage to prevention, care and treatment services to the following: |
| General population testing
|
In settings with a ≥ 2% (intermediate) or ≥ 5% (high) HCV antibody seroprevalence in the general population, it is recommended that all adults have access to and be offered HCV serological testing with linkage to prevention, care and treatment services. |
| Which serological assay to use |
To test for serological evidence of past or present infection in adults, adolescents and children (>18 months of age), an HCV serological assay (antibody or antibody/antigen) using either a rapid diagnostic test (RDT) or laboratory-based immunoassay formats that meet minimum safety, quality and performance standards (with regard to both analytical and clinical sensitivity and specificity) is recommended. • In settings where there is limited access to laboratory infrastructure and testing, and/or in populations where access to rapid testing would facilitate linkage to care and treatment, RDTs are recommended. (Strong recommendation, low/moderate quality of evidence) |
In a guideline pertaining to the screening, care, and treatment of people with chronic hepatitis C infection, the WHO has provided the following recommendations on hepatitis C screening:
- “Who to test for HCV infection?
- Focused testing in most-affected populations. In all settings (and regardless of whether delivered through facility- or community-based testing), it is recommended that serological testing for HCV antibody (anti-HCV)1 be offered with linkage to prevention, care and treatment services to the following individuals:
- Adults and adolescents from populations most affected by HCV infection (i.e., who are either part of a population with high HCV seroprevalence or who have a history of exposure and/or high-risk behaviours for HCV infection);
- Adults, adolescents and children with a clinical suspicion of chronic viral hepatitis (i.e. symptoms, signs, laboratory markers). (Strong recommendation, low quality of evidence)
- Note: Periodic retesting using HCV nucleic acid tests (NAT) should be considered for those with ongoing risk of acquisition or reinfection.
- General population testing. In settings with a ≥ 2% or ≥ 5% 4 HCV antibody seroprevalence in the general population, it is recommended that all adults have access to and be offered HCV serological testing with linkage to prevention, care and treatment services.
- General population testing approaches should make use of existing community- or facility based testing opportunities or programmes such as HIV or TB clinics, drug treatment services and antenatal clinics. (Conditional recommendation, low quality of evidence)
- Birth cohort testing. This approach may be applied to specific identified birth cohorts of older persons at higher risk of infection and morbidity within populations that have an overall lower general prevalence. (Conditional recommendation, low quality of evidence)”
- Focused testing in most-affected populations. In all settings (and regardless of whether delivered through facility- or community-based testing), it is recommended that serological testing for HCV antibody (anti-HCV)1 be offered with linkage to prevention, care and treatment services to the following individuals:
- “How to test for chronic HCV infection and monitor treatment response?
- Which serological assay to use? To test for serological evidence of past or present infection in adults, adolescents and children (> 18 months of age), an HCV serological assay (antibody or antibody/antigen) using either a rapid diagnostic test (RDT) or laboratory-based immunoassay formats that meet minimum safety, quality and performance standards (with regard to both analytical and clinical sensitivity and specificity) is recommended.
- In settings where there is limited access to laboratory infrastructure and testing, and/or in populations where access to rapid testing would facilitate linkage to care and treatment, RDTs are recommended. (Strong recommendation, low/moderate quality of evidence)
- Serological testing strategies. In adults and children older than 18 months, a single serological assay for initial detection of serological evidence of past or present infection is recommended prior to supplementary nucleic acid testing (NAT) for evidence of viraemic infection. (Conditional recommendation, low quality of evidence)
- Detection of viraemic infection
- Directly following a reactive HCV antibody serological test result, the use of quantitative or qualitative NAT for detection of HCV RNA is recommended as the preferred strategy to diagnose viraemic infection. (Strong recommendation, moderate/low quality of evidence)
- An assay to detect HCV core (p22) antigen, which has comparable clinical sensitivity to NAT, is an alternative to NAT to diagnose viraemic infection. (Conditional recommendation, moderate quality of evidence)
- Assessment of HCV treatment response
- Nucleic acid testing for qualitative or quantitative detection of HCV RNA should be used as the test of cure at 12 or 24 weeks (i.e., sustained virological response [SVR12 or SVR24]) after completion of antiviral treatment. (Conditional recommendation, moderate/low quality of evidence)” (WHO, 2018).
- Which serological assay to use? To test for serological evidence of past or present infection in adults, adolescents and children (> 18 months of age), an HCV serological assay (antibody or antibody/antigen) using either a rapid diagnostic test (RDT) or laboratory-based immunoassay formats that meet minimum safety, quality and performance standards (with regard to both analytical and clinical sensitivity and specificity) is recommended.
The WHO also includes a table which shows the populations with a high HCV prevalence or who have a history of HCV risk. The following groups are included:
- “Persons who have received medical or dental interventions in health-care settings where infection control practices are substandard
- Persons who have received blood transfusions prior to the time when serological testing of blood donors for HCV was initiated or in countries where serological testing of blood donations for HCV is not routinely performed
- People who inject drugs (PWID)
- Persons who have had tattoos, body piercing or scarification procedures done where infection control practices are substandard
- Children born to mothers infected with HCV
- Persons with HIV infection
- Persons who use/have used intranasal drugs
- Prisoners and previously incarcerated persons” (WHO, 2016)
The WHO also mentions liver function tests several times, stating that “A number of clinical considerations are important for the management of persons with chronic HCV infection”; further, “Pre-treatment evaluation of the risk of adverse events should be based on the patient’s clinical details, concomitant medications, and knowledge of treatment regimen to be administered. The potential for DDIs [drug-drug interactions] should be assessed before treatment, and a regimen that has a low risk of DDI selected. Standard laboratory tests that are assessed prior to treatment initiation include a full blood count (FBC), international normalized ratio (INR), renal function and liver function tests: ALT, AST, bilirubin, albumin and alkaline phosphatase” (WHO, 2016).
The WHO also mentions that “in persons with HCV infection being treated for TB, it is important to monitor liver function tests” and that “Baseline liver function tests for individuals with chronic liver disease are encouraged prior to initiating treatment for latent TB infection. For individuals with abnormal baseline test results, routine periodic laboratory testing should be carried out during the treatment of latent TB infection” (WHO, 2016).
The recommendations of the WHO for assays and strategies regarding hepatitis C testing are summarized in their table, captured below (WHO, 2017):

Hepatitis B
The below table details the populations who should be tested for chronic hepatitis B infection, according to the WHO (WHO, 2017):
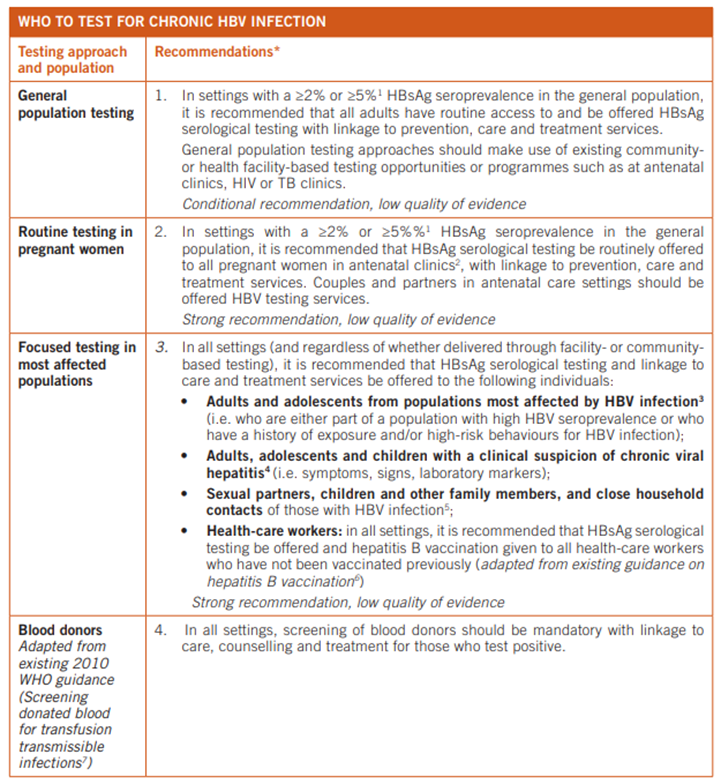
Similarly, the recommendations of the WHO for assays and strategies regarding hepatitis B testing are summarized in their table, captured below (WHO, 2017):
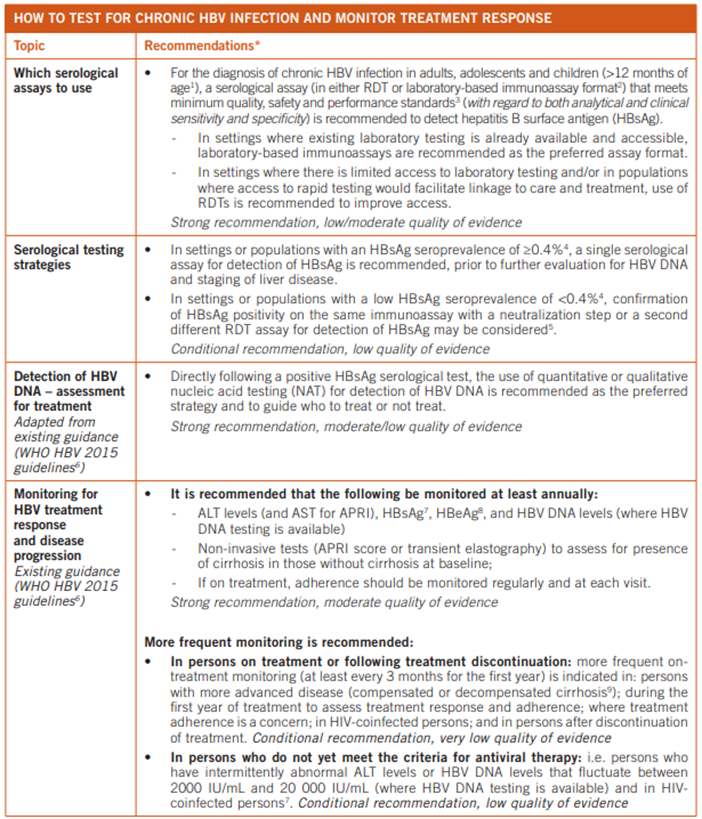
Hepatitis D
For the diagnosis of hepatitis D, the WHO states that HDV infection is “diagnosed by high levels of anti-HDV immunoglobulin G (IgG) and immunoglobulin M (IgM), and confirmed by detection of HDV RNA in serum. However, HDV diagnostics are not widely available and there is no standardization for HDV RNA assays, which are used for monitoring response to antiviral therapy”(WHO, 2023).
American Gastroenterological Association (AGA)
Hepatitis B
“The AGA recommends screening for HBV (HBsAg and anti-HBc, followed by a sensitive HBV DNA test if positive) in patients at moderate or high risk who will undergo immunosuppressive drug therapy. (Strong recommendation; Moderate-quality evidence) The AGA suggests against routinely screening for HBV in patients who will undergo immunosuppressive drug therapy and are at low risk. (Weak recommendation; Moderate-quality evidence) Comments: Patients in populations with a baseline prevalence likely exceeding 2% for chronic HBV should be screened according to Centers for Disease Control and Prevention and US Preventive Services Task Force recommendations” (Reddy et al., 2015).
Hepatitis C
The AGA released best practice statements for care of patients with chronic HCV that have achieved a sustained virologic response (SVR).
- “SVR should be confirmed by undetectable HCV RNA at 12 weeks after completion of an all-oral DAA treatment regimen.”
- “Routine confirmation of SVR at 48 weeks post end of treatment is recommended. Testing for HCV RNA at 24 weeks post treatment should be considered on an individual patient basis.”
- “Routine testing for HCV RNA beyond 48 weeks after end of treatment to evaluate for late virologic relapse is not supported by available evidence; periodic testing for HCV RNA is recommended for patients with ongoing risk factors for reinfection” (Jacobson et al., 2017).
The AGA has also released a “pathway” for HCV treatment (an algorithm).
Prior to treatment, the AGA recommends identifying the HCV genotype, as well as taking a hepatic function panel (defined as albumin, total and direct bilirubin, alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase).
For all three lengths of treatment courses (8, 12, 16 weeks), the AGA recommends assessing viral load and liver function (the same hepatic panel listed above) (Kanwal et al., 2017).
European Association for the Study of the Liver (EASL)
Hepatitis C
The EASL released guidelines on treatment of hepatitis C. The EASL recommends:
- “Screening strategies for HCV infection should be defined according to the local epidemiology of HCV infection, ideally within the framework of local, regional or national action plans.
- Liver disease severity must be assessed prior to therapy.
- Rapid diagnostic tests using serum, plasma, fingerstick whole blood or crevicular fluid (saliva) as matrices can be used instead of classical EIAs as point-of-care tests to facilitate anti-HCV antibody screening and improve access to care.
- “It is still useful to determine the HCV genotype and subtype where such determination is available and does not limit access to care, to identify patients who may benefit from treatment tailoring. However, “testing for HCV resistance prior to treatment is not recommended” (EASL, 2020).
Hepatitis B
The EASL states that “The initial evaluation of a subject with chronic HBV infection should include a complete history, a physical examination, assessment of liver disease activity and severity and markers of HBV infection (Fig. 1). In addition, all first-degree relatives and sexual partners of subjects with chronic HBV infection should be advised to be tested for HBV serological markers (HBsAg, anti-HBs, anti-HBc) and to be vaccinated if they are negative for these markers.”
“The assessment of the severity of liver disease is important to identify patients for treatment and HCC surveillance. It is based on a physical examination and biochemical parameters (aspartate aminotransferase [AST] and ALT, gamma-glutamyl transpeptidase [GGT], alkaline phosphatase, bilirubin, and serum albumin and gamma globulins, full blood count and prothrombin time). An abdominal hepatic ultrasound is recommended in all patients. A liver biopsy or a non-invasive test should be performed to determine disease activity in cases where biochemical and HBV markers reveal inconclusive results.”
“HBeAg and anti-HBe detection are essential for the determination of the phase of chronic HBV infection.”
“Measurement of HBV DNA serum level is essential for the diagnosis, establishment of the phase of the infection, the decision to treat and subsequent monitoring of patients.”
“Serum HBsAg quantification can be useful, particularly in HBeAg-negative chronic HBV infection and in patients to be treated with interferon-alfa (IFNα).”
“HBV genotype is not necessary in the initial evaluation, although it may be useful for selecting patients to be treated with IFNα offering prognostic information for the probability of response to IFNα therapy and the risk of HCC.”
“Co-morbidities, including alcoholic, autoimmune, metabolic liver disease with steatosis or steatohepatitis and other causes of chronic liver disease should be systematically excluded including co-infections with hepatitis D virus (HDV), hepatitis C virus (HCV) and HIV.”
“Testing for antibodies against hepatitis A virus (anti-HAV) should be performed, and patients with negative anti-HAV should be advised to be vaccinated against HAV.”
“Screening for HBsAg in the first trimester of pregnancy is strongly recommended (Evidence level 1, grade of recommendation 1)” (Lampertico et al., 2017).
Hepatitis D
In the clinical practice guidelines for Hepatitis D, the EASL provided the following recommendations:
- “Screening for anti-HDV antibodies should be performed with a validated assay at least once in all HBsAg-positive individuals (LoE 3, strong recommendation, strong consensus).
- Re-testing for anti-HDV antibodies should be performed in HBsAg-positive individuals whenever clinically indicated (e.g., in case of aminotransferase flares, or acute decompensation of chronic liver disease) (LoE 3, strong recommendation, strong consensus), and may be performed yearly in those remaining at risk of infection (LoE 5, weak recommendation, strong consensus)
- HDV RNA should be tested in all anti-HDV-positive individuals using a standardised and sensitive reverse transcription PCR assay to diagnose active HDV infection (LoE 2, strong recommendation, strong consensus).
- In patients with acute hepatitis, anti-HBc IgM should be used to distinguish individuals with HBV/HDV coinfection from HBsAg-positive individuals superinfected with HDV (LoE 3; strong recommendation, consensus).
- HBV e antigen (HBeAg)/anti-HBe status and HBV DNA levels should be tested because the presence of active HBV infection may worsen the outcome of hepatitis D (LoE 3; strong recommendation, consensus)” (Brunetto et al., 2023).
In this clinical guideline, the EASL notes the following concerning quantitative RNA monitoring for HDV: “Preliminary reports suggest that viral load correlates with disease activity and progression; however, further studies with standardised assays are required to confirm these findings and define the prognostic role of quantitative HDV RNA monitoring in untreated patients” (Brunetto et al., 2023).
Indian Health Services (IHS)
Indian Health Services published recommendations on Hepatitis C screening. IHS recommends using an anti-HCV antibody test such as a point-of-care test on a fingerstick capillary or venipuncture whole-blood sample or a laboratory-based HCV ELISA test on a serum sample. IHS recommends screening the following patients:
- "Adults 18 years and older, including people with diabetes, at least once for HCV infection, regardless of their risk factors.
- All pregnant persons, regardless of age, during each pregnancy.
- People at higher risk of HCV exposure” (IHS, 2021).
IHS also provides guidance on how to diagnose a chronic HCV infection:
- "For individuals with a positive HCV antibody screening test result, perform the laboratory-based HCV RNA PCR test to confirm the presence of HCV.
- The presence of HCV indicates active infection. These individuals should be referred for direct acting anti-viral (DAA) agents treatment.
- The absence of HCV indicates no active infection.
- For individuals with a negative HCV antibody test result who might have been exposed to HCV within the previous six months, perform an HCV RNA PCR or follow-up HCV antibody test at least six months after exposure” (IHS, 2021).
Regarding hepatitis B, the IHS suggests that
“People who inject drugs illicitly, including participants in substance abuse treatment programs, should be offered screening and counseling for chronic HBV infection. Testing should include a serologic assay for hepatitis B surface antigen (HBsAg) offered as a part of routine care, and if the result is positive, be accompanied by appropriate counseling and referral for recommended clinical evaluation and care. Previous and current sex partners and household and needle-sharing contacts of HBsAg-positive persons should be identified. Unvaccinated sex partners and household and needle-sharing contacts should be tested for HBsAg and for antibody to the hepatitis B core antigen (anti-HBc) or antibody to the hepatitis B surface antigen (anti-HBsAg)” (IHS, 2022).
References
- AASLD-IDSA. (2015). Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology, 62(3), 932-954. https://doi.org/10.1002/hep.27950
- AASLD-IDSA. (2022a, 09/29/2021). HCV Testing and Linkage to Care. https://www.hcvguidelines.org/evaluate/testing-and-linkage
- AASLD-IDSA. (2022b, 10/24/2022). Management of Acute Infection. https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/AASLD-IDSA_HCVGuidance_October_24_2022.pdf
- AASLD-IDSA. (2023a, December 19). HCV in Pregnancy. https://www.hcvguidelines.org/unique-populations/pregnancy
- AASLD-IDSA. (2023b, December 19). Monitoring Patients Who Are Starting HCV Treatment, Are on Treatment, or Have Completed Therapy. https://www.hcvguidelines.org/evaluate/monitoring
- AASLD. (2023). Don’t repeat hepatitis C virus (HCV) antibody testing in patients with a previous positive (HCV) test. Instead, order hepatitis C viral load testing for assessment of active versus resolved infection. https://www.aafp.org/pubs/afp/collections/choosing-wisely/420.html
- Ansaldi, F., Orsi, A., Sticchi, L., Bruzzone, B., & Icardi, G. (2014). Hepatitis C virus in the new era: perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World J Gastroenterol, 20(29), 9633-9652. https://doi.org/10.3748/wjg.v20.i29.9633
- Bailey, J. R., Barnes, E., & Cox, A. L. (2019). Approaches, Progress, and Challenges to Hepatitis C Vaccine Development. Gastroenterology, 156(2), 418-430. https://doi.org/10.1053/j.gastro.2018.08.060
- Bhattacharya, D., Aronsohn, A., Price, J., & Lo Re, V. (2023). Hepatitis C Guidance 2023 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. https://doi.org/10.1093/cid/ciad319
- BioMérieux. (2022). VIDAS® Hepatitis panel. https://www.biomerieux.com/corp/en/our-offer/clinical-products/vidas-hepatitis-panel.html
- Brunetto, M. R., Ricco, G., Negro, F., Wedemeyer, H., Yurdaydin, C., Asselah, T., Papatheodoridis, G., Gheorghe, L., Agarwal, K., Farci, P., & Buti, M. (2023). EASL Clinical Practice Guidelines on hepatitis delta virus. J Hepatol, 79(2), 433-460. https://doi.org/10.1016/j.jhep.2023.05.001
- Catlett, B., Bajis, S., Starr, M., Dore, G. J., Hajarizadeh, B., Cunningham, P. H., Applegate, T. L., & Grebely, J. (2021). Evaluation of the Aptima HCV Quant Dx Assay for Hepatitis C Virus RNA Detection from Fingerstick Capillary Dried Blood Spot and Venepuncture-Collected Samples. J Infect Dis, 223(5), 818-826. https://doi.org/10.1093/infdis/jiaa442
- CDC. (2012). Recommendations for the Identification of Chronic Hepatitis C Virus Infection Among Persons Born During 1945–1965. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm
- CDC. (2015). Community Outbreak of HIV Infection Linked to Injection Drug Use of Oxymorphone — Indiana, 2015. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6416a4.htm
- CDC. (2018). Surveillance for Viral Hepatitis – United States, 2018. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm
- CDC. (2020a). The ABCs of Hepatitis – for Health Professionals. Centers for Disease Control and Prevention. https://www.cdc.gov/hepatitis/resources/professionals/pdfs/ABCTable.pdf
- CDC. (2020b). Hepatitis C. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm
- CDC. (2020c, 03/09/2020). Hepatitis D Questions and Answers for Health Professionals. https://www.cdc.gov/hepatitis/hdv/hdvfaq.htm#d1
- CDC. (2020d). Testing and Clinical Management of Health Care Personnel Potentially Exposed to Hepatitis C Virus — CDC Guidance, United States, 2020. https://www.cdc.gov/mmwr/volumes/69/rr/rr6906a1.htm
- CDC. (2023a, December 19, 2023). Clinical Screening and Diagnosis for Hepatitis C. https://www.cdc.gov/hepatitis-c/hcp/diagnosis-testing/
- CDC. (2023b). Health Care Providers and Viral Hepatitis. https://www.cdc.gov/hepatitis/populations/healthcaresettings.htm
- CDC. (2023c, March 30, 2022). Recommendations for Routine Testing and Follow-up for Chronic Hepatitis B Virus (HBV) Infection. Centers for Disease Control and Prevention. https://www.cdc.gov/hepatitis/hbv/HBV-RoutineTesting-Followup.htm#print
- CDC. (2024a, January 11, 2024). Clinical Overview of Hepatitis A. https://www.cdc.gov/hepatitis-a/hcp/clinical-overview/
- CDC. (2024b, March 6, 2024). Clinical Testing and Diagnosis for Hepatitis B. https://www.cdc.gov/hepatitis-b/hcp/diagnosis-testing/
- Chen, Y., Ji, H., Shao, J., Jia, Y., Bao, Q., Zhu, J., Zhang, L., & Shen, Y. (2019). Different Hepatitis C Virus Infection Statuses Show a Significant Risk of Developing Type 2 Diabetes Mellitus: A Network Meta-Analysis. Dig Dis Sci. https://doi.org/10.1007/s10620-019-05918-7
- Chevaliez, S., Wlassow, M., Volant, J., Roudot-Thoraval, F., Bachelard, A., Poiteau, L., Trabut, J. B., Hézode, C., Bourdel, A., & Dominguez, S. (2020). Assessing Molecular Point-of-Care Testing and Dried Blood Spot for Hepatitis C Virus Screening in People Who Inject Drugs. Open Forum Infect Dis, 7(6), ofaa196. https://doi.org/10.1093/ofid/ofaa196
- Chopra, S. (2024, February 7). Clinical manifestations and natural history of chronic hepatitis C virus infection. https://www.uptodate.com/contents/clinical-manifestations-and-natural-history-of-chronic-hepatitis-c-virus-infection
- Chopra, S., & Arora, S. (2024a, January 24). Patient evaluation and selection for antiviral therapy for chronic hepatitis C virus infection. https://www.uptodate.com/contents/patient-evaluation-and-selection-for-antiviral-therapy-for-chronic-hepatitis-c-virus-infection
- Chopra, S., & Arora, S. (2024b, April 10, 2024). Screening and diagnosis of chronic hepatitis C virus infection. https://www.uptodate.com/contents/screening-and-diagnosis-of-chronic-hepatitis-c-virus-infection
- Chopra, S., & Pockros, P. J. (2024, April 5, 2024). Overview of the management of chronic hepatitis C virus infection. https://www.uptodate.com/contents/overview-of-the-management-of-chronic-hepatitis-c-virus-infection
- EASL. (2020). EASL recommendations on treatment of Hepatitis C 2020. https://easl.eu/wp-content/uploads/2020/10/EASL-recommendations-on-treatment-of-hepatitis-C.pdf
- Fleurence, R. L., & Collins, F. S. (2023). A National Hepatitis C Elimination Program in the United States: A Historic Opportunity. Jama, 329(15), 1251-1252. https://doi.org/10.1001/jama.2023.3692
- Ghany, M. G., & Morgan, T. R. (2020). Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology, 71(2), 686-721. https://doi.org/10.1002/hep.31060
- Hagan, H., Campbell, J., Thiede, H., Strathdee, S., Ouellet, L., Kapadia, F., Hudson, S., & Garfein, R. S. (2006). Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Rep, 121(6), 710-719. https://doi.org/10.1177/003335490612100611
- Health, L. (2023). Hepatitis Chronic Panel. https://www.testmenu.com/legacylab/Tests/1103205
- IHS. (2021). Hepatitis C and Tuberculosis Screening. https://www.ihs.gov/diabetes/clinician-resources/soc/hepc-tb-screening/
- IHS. (2022). Harm Reduction - Infectious Diseases. https://www.ihs.gov/opioids/harmreduction/infectiousdiseases/
- Inc., S. D. (2023). SD BIOLINE HCV. https://maxanim.com/content/abbott/sd-bioline-hcv.pdf
- Inoue, J., Kanno, A., Wakui, Y., Miura, M., Kobayashi, T., Morosawa, T., Kogure, T., Kakazu, E., Ninomiya, M., Fujisaka, Y., Umetsu, T., Takai, S., Nakamura, T., & Shimosegawa, T. (2017). Identification of Genotype 2 HCV in Serotype-1 Hepatitis C Patients Unresponsive to Daclatasvir plus Asunaprevir Treatment. Tohoku J Exp Med, 241(1), 21-28. https://doi.org/10.1620/tjem.241.21
- Jacobson, I. M., Lim, J. K., & Fried, M. W. (2017). American Gastroenterological Association Institute Clinical Practice Update-Expert Review: Care of Patients Who Have Achieved a Sustained Virologic Response After Antiviral Therapy for Chronic Hepatitis C Infection. Gastroenterology, 152(6), 1578-1587. https://doi.org/10.1053/j.gastro.2017.03.018
- JMitra&Co. (2015). HCV TRI-DOT. http://www.jmitra.co.in/ourdivision/diagnosticdivision/rapidtestkits/hcvrange/hcv_tri_dot.aspx
- Kanwal, F., Bacon, B. R., Beste, L. A., Brill, J. V., Gifford, A. L., Gordon, S. C., Horberg, M. A., Manthey, J. G., Reau, N., Rustgi, V. K., & Younossi, Z. M. (2017). Hepatitis C Virus Infection Care Pathway - A Report From the American Gastroenterological Association Institute HCV Care Pathway Work Group. Gastroenterology, 152(6), 1588-1598. https://doi.org/10.1053/j.gastro.2017.03.039
- Keles, E., Hassan-Kadle, M. A., Osman, M. M., Eker, H. H., Abusoglu, Z., Baydili, K. N., & Osman, A. M. (2021). Clinical characteristics of acute liver failure associated with hepatitis A infection in children in Mogadishu, Somalia: a hospital-based retrospective study. BMC Infect Dis, 21(1), 890. https://doi.org/10.1186/s12879-021-06594-7
- Krist, A. H., Davidson, K. W., Mangione, C. M., Barry, M. J., Cabana, M., Caughey, A. B., Donahue, K., Doubeni, C. A., Epling, J. W., Jr., Kubik, M., Ogedegbe, G., Owens, D. K., Pbert, L., Silverstein, M., Simon, M. A., Tseng, C. W., & Wong, J. B. (2020). Screening for Hepatitis B Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. Jama, 324(23), 2415-2422. https://doi.org/10.1001/jama.2020.22980
- Lai, M., & Chopra, S. (2024, February 1). Hepatitis A virus infection in adults: Epidemiology, clinical manifestations, and diagnosis. https://www.uptodate.com/contents/hepatitis-a-virus-infection-in-adults-epidemiology-clinical-manifestations-and-diagnosis
- Lampertico, P., Agarwal, K., Berg, T., Buti, M., Janssen, H. L. A., Papatheodoridis, G., Zoulim, F., & Tacke, F. (2017). EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol, 67(2), 370-398. https://doi.org/https://doi.org/10.1016/j.jhep.2017.03.021
- Lexidrug. (2024). Ledipasvir and sofosbuvir: Drug information. https://www.uptodate.com/contents/ledipasvir-and-sofosbuvir-drug-information
- Linthicum, M. T., Gonzalez, Y. S., Mulligan, K., Moreno, G. A., Dreyfus, D., Juday, T., Marx, S. E., Lakdawalla, D. N., Edlin, B. R., & Brookmeyer, R. (2016). Value of expanding HCV screening and treatment policies in the United States. Am J Manag Care, 22(6 Spec No.), Sp227-235. https://www.ajmc.com/journals/issue/2016/2016-5-vol22-sp/value-of-expanding-hcv-screening-and-treatment-policies-in-the-united-states?p=1
- Lok, A. S. (2021, July 30). Hepatitis B virus: Overview of management. https://www.uptodate.com/contents/hepatitis-b-virus-overview-of-management
- Messina, J. P., Humphreys, I., Flaxman, A., Brown, A., Cooke, G. S., Pybus, O. G., & Barnes, E. (2015). Global distribution and prevalence of hepatitis C virus genotypes. Hepatology, 61(1), 77-87. https://doi.org/10.1002/hep.27259
- Moreno, G. A., Mulligan, K., Huber, C., Linthicum, M. T., Dreyfus, D., Juday, T., Marx, S. E., Gonzalez, Y. S., Brookmeyer, R., & Lakdawalla, D. N. (2016). Costs and spillover effects of private insurers' coverage of hepatitis C treatment. Am J Manag Care, 22(6 Spec No.), Sp236-244. https://www.ajmc.com/journals/issue/2016/2016-5-vol22-sp/costs-and-spillover-effects-of-private-insurers-coverage-of-hepatitis-c-treatment?p=1
- Muir, A. J., & Graham, C. S. (2024, January 24). Management of chronic hepatitis C virus infection: Initial antiviral therapy in adults. https://www.uptodate.com/contents/management-of-chronic-hepatitis-c-virus-infection-initial-antiviral-therapy-in-adults
- OraSure. (2013). OraQuick® HCV test https://www.orasure.com/products-infectious/products-infectious-oraquick-hcv.asp
- Owens, D. K., Davidson, K. W., Krist, A. H., Barry, M. J., Cabana, M., Caughey, A. B., Donahue, K., Doubeni, C. A., Epling, J. W., Jr., Kubik, M., Ogedegbe, G., Pbert, L., Silverstein, M., Simon, M. A., Tseng, C. W., & Wong, J. B. (2020). Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. Jama. https://doi.org/10.1001/jama.2020.1123
- Razavi, H., Waked, I., Sarrazin, C., Myers, R. P., Idilman, R., Calinas, F., Vogel, W., Mendes Correa, M. C., Hezode, C., Lazaro, P., Akarca, U., Aleman, S., Balik, I., Berg, T., Bihl, F., Bilodeau, M., Blasco, A. J., Brandao Mello, C. E., Bruggmann, P., . . . Estes, C. (2014). The present and future disease burden of hepatitis C virus (HCV) infection with today's treatment paradigm. J Viral Hepat, 21 Suppl 1, 34-59. https://doi.org/10.1111/jvh.12248
- Reddy, K. R., Beavers, K. L., Hammond, S. P., Lim, J. K., & Falck-Ytter, Y. T. (2015). American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology, 148(1), 215-219; quiz e216-217. https://doi.org/10.1053/j.gastro.2014.10.039
- Rein, D. B., Smith, B. D., Wittenborn, J. S., Lesesne, S. B., Wagner, L. D., Roblin, D. W., Patel, N., Ward, J. W., & Weinbaum, C. M. (2012). The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med, 156(4), 263-270. https://doi.org/10.7326/0003-4819-156-4-201202210-00378
- Saeed, Y. A., Phoon, A., Bielecki, J. M., Mitsakakis, N., Bremner, K. E., Abrahamyan, L., Pechlivanoglou, P., Feld, J. J., Krahn, M., & Wong, W. W. L. (2020). A Systematic Review and Meta-Analysis of Health Utilities in Patients With Chronic Hepatitis C. Value Health, 23(1), 127-137. https://doi.org/10.1016/j.jval.2019.07.005
- Simmonds, P. (2001). Reconstructing the origins of human hepatitis viruses. Philos Trans R Soc Lond B Biol Sci, 356(1411), 1013-1026. https://doi.org/10.1098/rstb.2001.0890
- Spach. (2020). Hepatitis C Diagnostic Testing. https://www.hepatitisc.uw.edu/go/screening-diagnosis/diagnostic-testing/core-concept/all
- Spenatto, N., Boulinguez, S., Mularczyk, M., Molinier, L., Bureau, C., Saune, K., & Viraben, R. (2013). Hepatitis B screening: who to target? A French sexually transmitted infection clinic experience. J Hepatol, 58(4), 690-697. https://doi.org/10.1016/j.jhep.2012.11.044
- Su, S., Wong, W. C., Zou, Z., Cheng, D. D., Ong, J. J., Chan, P., Ji, F., Yuen, M. F., Zhuang, G., Seto, W. K., & Zhang, L. (2022). Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Glob Health, 10(2), e278-e287. https://doi.org/10.1016/s2214-109x(21)00517-9
- Teo, E.-K., & Lok, A. S. F. (2022, 09/21/2022). Epidemiology, transmission, and prevention of hepatitis B virus infection. https://www.uptodate.com/contents/epidemiology-transmission-and-prevention-of-hepatitis-b-virus-infection
- Terrault, N. A., Lok, A. S. F., McMahon, B. J., Chang, K. M., Hwang, J. P., Jonas, M. M., Brown, R. S., Jr., Bzowej, N. H., & Wong, J. B. (2018). Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology, 67(4), 1560-1599. https://doi.org/10.1002/hep.29800
- Vetter, B. N., Reipold, E. I., Ongarello, S., Audu, R., Ige, F. A., Alkhazashvili, M., Chitadze, N., Vanroye, F., De Weggheleire, A., An, S., & Fransen, K. (2022). Sensitivity and Specificity of Rapid Diagnostic Tests for Hepatitis C Virus With or Without HIV Coinfection: A Multicentre Laboratory Evaluation Study. The Journal of Infectious Diseases. https://doi.org/10.1093/infdis/jiaa389
- Wandeler, G., Schlauri, M., Jaquier, M. E., Rohrbach, J., Metzner, K. J., Fehr, J., Ambrosioni, J., Cavassini, M., Stockle, M., Schmid, P., Bernasconi, E., Keiser, O., Salazar-Vizcaya, L., Furrer, H., Rauch, A., Aubert, V., Battegay, M., Bernasconi, E., Boni, J., . . . Yerly, S. (2015). Incident Hepatitis C Virus Infections in the Swiss HIV Cohort Study: Changes in Treatment Uptake and Outcomes Between 1991 and 2013. Open Forum Infect Dis, 2(1), ofv026. https://doi.org/10.1093/ofid/ofv026
- WHO. (2016). Guidelines for the Screening, Care and Treatment of Persons With Chronic Hepatitis C Infection World Health Organization Copyright (c) World Health Organization 2016. https://iris.who.int/bitstream/handle/10665/205035/9789241549615_eng.pdf
- WHO. (2017). Guidelines on Hepatitis B and C Testing. https://iris.who.int/bitstream/handle/10665/254621/9789241549981-eng.pdf
- WHO. (2018). Guidelines for the Care and Treatment of Persons Diagnosed With Chronic Hepatitis C Infection https://iris.who.int/bitstream/handle/10665/273174/9789241550345-eng.pdf
- WHO. (2023, 07/20/2023). Hepatitis D. https://www.who.int/news-room/fact-sheets/detail/hepatitis-d
Coding Section
| Codes | Number | Description |
| 86692 | Antibody; hepatitis, delta agent | |
| CPT | 86704 | Hepatitis B core antibody (HBcAb); total |
| 86705 | Hepatitis B core antibody (HBcAb); IgM antibody | |
| 86706 | Hepatitis B surface antibody (HBsAb) | |
| 86708 | Hepatitis A antibody (HAAb) | |
| 86709 | Hepatitis A antibody (HAAb), IgM antibody | |
| 86803 | Hepatitis C antibody | |
| 86804 | Hepatitis C antibody; confirmatory test (eg, immunoblot) | |
| 87340 | Infectious agent antigen detection by immunoassay technique, (e.g., enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], fluorescence immunoassay [FIA], immunochemiluminometric assay [IMCA]) qualitative or semiquantitative; hepatitis B surface antigen (HBsAg) | |
| 87341 | Infectious agent antigen detection by immunoassay technique, (e.g. enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], fluorescence immunoassay [FIA], immunochemiluminometric assay [IMCA]) qualitative or semiquantitative; hepatitis B surface antigen (HBsAg) neutralization | |
| 87380 | Infectious agent antigen detection by immunoassay technique, (eg, enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], fluorescence immunoassay [FIA], immunochemiluminometric assay [IMCA]) qualitative or semiquantitative; hepatitis, delta agentInfectious agent antigen detection by immunoassay technique, (eg, enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], fluorescence immunoassay [FIA], immunochemiluminometric assay [IMCA]) qualitative or semiquantitative; hepatitis, delta agent | |
| 87516 | Infectious agent detection by nucleic acid (DNA or RNA); hepatitis B virus, amplified probe technique | |
| 87517 | Infectious agent detection by nucleic acid (DNA or RNA); hepatitis B virus, quantification | |
| 87520 | Infectious agent detection by nucleic acid (DNA or RNA); hepatitis C, direct probe technique | |
| 87521 | Infectious agent detection by nucleic acid (DNA or RNA); hepatitis C, amplified probe technique, includes reverse transcription when performed | |
| 87522 | Infectious agent detection by nucleic acid (DNA or RNA); hepatitis C, quantification, includes reverse transcription when performed | |
| 87523 | Infectious agent detection by nucleic acid (DNA or RNA); hepatitis D (delta), quantification, including reverse transcription, when performed | |
| 87799 | Infectious agent detection by nucleic acid (DNA or RNA), not otherwise specified; quantification, each organism | |
| 87902 | Infectious agent genotype analysis by nucleic acid (DNA or RNA); Hepatitis C virus | |
| G0472 | Hepatitis C antibody screening, for individual at high risk and other covered indication(s) | |
| G0499 |
Hepatitis b screening in non-pregnant, high risk individual includes hepatitis b surface antigen (hbsag), antibodies to hbsag (anti-hbs) and antibodies to hepatitis b core antigen (anti-hbc), and is followed by a neutralizing confirmatory test, when performed, only for an initially reactive hbsag result | |
| ICD 10 Diagnoses Code | B18.2 | Chronic hepatitis C |
| E920.5 | Accidents caused by hypodermic needle | |
| F11.10-F11.99 | Opioid related disorders (injecting drug users) | |
| F13.10-F13.99 | Sedative, hypnotic, or anxiolytic related disorders (injecting drug users) | |
| F14.10-F14.99 | Cocaine related disorders (injecting drug users) | |
| F19.20 | Other psychoactive substance dependence, uncomplicated | |
| K75.2 | Nonspecific reactive hepatitis | |
| K75.81 | Nonalcoholic steatohepatitis (NASH) | |
| K76.81-K76.89 | Other specified diseases of liver | |
| K76.9 | Liver disease, unspecified | |
| L81.8 | Other specified disorders of pigmentation, Tattoo pigmentation | |
| N25.0 | Renal osteodystrophy | |
| R74.0 | Nonspecific elevation of levels of transaminase and lactic acid dehydrogenase (LDH) | |
| R74.8 |
Abnormal levels of other serum enzymes | |
| R74.9 | Abnormal serum enzyme level, unspecified | |
| T80.61XA - T80.61XS | Complications following infusion, transfusion and therapeutic injection | |
| W46.1XXA-W46.1XXS | Contact with contaminated hypodermic needle | |
| Z11.59 | Encounter for screening for other viral diseases | |
| Z20.5 | Contact with and (suspected) exposure to viral hepatitis | |
| Z21, B20, B97.35 | HIV | |
| Z65.1 | Imprisonment and other incarceration | |
| Z72.51-Z72.53 | High risk sexual behavior | |
| Z72.89 | Other problems related to lifestyle | |
| Z77.21 | Contact with and (suspected) exposure to potentially hazardous body fluids | |
| Z79.01 | Long term (current) use of anticoagulants | |
| Z79.899 | Other long term (current) drug therapy | |
| Z99.2 | Dependence on renal dialysis |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2016 Forward
| 10/16/2024 | Annual review, policy being updated to include coverage statements specific to hepatitis a and hepatitis d. for clarity and consistency. Also updating rationale and references. Numerous coding additions. |
| 01/24/2024 | Annual review, updating policy to add criteria for HCV testing in perinatally exposed infants who are 2-27 months old. Also updating rationale and references. |
| 11/06/2023 | Interim review, change review month to October. Updating entire policy for clarity and consistency. Policy criteria is being broken into sections that address Hepatitis B and C separately for ease of use. Some criteria updates have been made in relations to new CDC guidance. |
| 04/06/2023 | Annual review with major revisions. Policy title change to Hepatitis Testing and criteria regarding Hepatitis B testing has been added. Updating coding, rationale, description and references. |
| 07/27/2022 | Annual review, updating policy to include verbiage regarding pregnant individuals, changing age range for once in a lifetime Hepatitis C screening, high risk sexual behavior and one time exposure. Also updating description, rationale and references. |
| 07/28/2021 |
Annual review,updating policy to include clarification of once in a lifetime tesing in #1, adding age and illicit intranasal or injectable drug use to #2, adding #3 related to ongoing risk factors. Also updating rationale and references. |
| 07/02/2020 |
Annual review, updating policy to align with CDC guidelines that were updated in 2020. No other changes. |
| 07/11/2019 |
Annual review, no change to policy intent. |
| 07/18/2018 |
Interim review to change review date to July. No other changes made. |
| 02/14/2018 |
Annual review, updating policy verbiage for clarity and to address testing frequency for HCV. Also expanding ICD10 code range. |
| 06/19/2017 |
Updated coding section. No other changes. |
| 04/26/2017 |
Updated category to Laboratory. No other changes. |
| 01/04/2017 |
Annual review, no change to policy intent. |
| 01/07/2016 |
NEW POLICY |