Testing for Vector-Borne Infections - CAM 210
Description
Arthropod vectors, including mosquitoes, ticks, fleas, and mites, that feed on vertebrate hosts can spread bacteria, protozoa, and viruses during feeding to their susceptible host, resulting in a variety of infections and diseases. Arboviruses (arthropod-borne viruses) include Zika virus, West Nile virus (WNV), chikungunya virus, dengue virus (DENV), yellow fever virus (YFV), and Colorado tick fever virus (CTF) to name a few. Malaria and babesiosis are both conditions caused by arthropod-borne protozoan parasites, Plasmodium and Babesia, respectively. Conditions caused by arthropod-borne bacteria include rickettsial diseases, ehrlichiosis, anaplasmosis, and Lyme disease, as well as other Borrelia-associated disorders (Calisher, 1994; CDC, 2024s). Isolation, identification, and characterization of these various infections depend on the causative agent. Identification methods may include culture testing, microscopy, and staining techniques; moreover, molecular testing, such as nucleic acid amplification testing (NAAT), and serologic testing, including immunofluorescence antibody assays and enzyme-linked immunosorbent assays (ELISA), can be used for laboratory diagnosis (Miller et al., 2024).
For Lyme disease and testing for Borrelia burgdorferi, please see CAM 159 Lyme Disease.
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
- For individuals suspected of having babesiosis (see Note 1), the use of a Giemsa- or Wright-stain of a blood smear or nucleic acid amplification testing (NAAT) is considered MEDICALLY NECESSARY.
- For individuals suspected of having babesiosis (see Note 1), the use of either an IgG or IgM indirect immunofluorescence antibody (IFA) assay for Babesia is considered NOT MEDICALLY NECESSARY.
- For individuals suspected of having a relapsing fever caused by a Borrelia spp., the following testing is considered MEDICALLY NECESSARY:
- For individuals suspected of having hard tick relapsing fever (HTRF) (see Note 2): serologic assays to detect Borrelia antibodies or PCR testing to detect Borrelia miyamotoi.
- For individuals suspected of having louse-borne relapsing fever (LBRF) (see Note 3): peripheral blood smear microscopy or PCR testing to detect Borrelia recurrentis.
- For individuals suspected of having a soft tick relapsing fever (STRF)/tickborne relapsing fever (TBRF) (see Note 4): dark-field microscopy of a peripheral blood smear, microscopy of a Wright- or Giemsa-stained blood smear, PCR testing to detect Borrelia spp., or serologic assays to detect Borrelia antibodies.
- For individuals suspected of having a relapsing fever caused by a Borrelia spp., culture testing for Borrelia is considered NOT MEDICALLY NECESSARY.
- For individuals suspected of having chikungunya (see Note 5), the use of viral culture for diagnosis, NAAT for the presence of chikungunya in a serum sample, or IFA assay for IgM antibodies during both the acute and convalescent phases is considered MEDICALLY NECESSARY.
- For individuals suspected of having Colorado tick fever (CTF) (see Note 6), the use of PCR testing or IFA for CTF-specific IgM antibodies is considered MEDICALLY NECESSARY.
- For the detection of dengue virus (DENV), the use of NAAT, IgM antibody capture ELISA (MAC-ELISA), or NS1 ELISA, as well as a confirmatory plaque reduction neutralization test for DENV, is considered MEDICALLY NECESSARY in the following individuals:
- For individuals suspected of having a DENV infection (see Note 7).
- For individuals who are symptomatic for Zika virus infection (see Note 8).
- For individuals suspected of having DENV (see Note 7), the use of IgG ELISA or hemagglutination testing is considered NOT MEDICALLY NECESSARY.
- For individuals suspected of having ehrlichiosis and/or anaplasmosis (see Note 8), the use of NAAT of whole blood, IFA assay for IgG antibodies, or microscopy for morulae detection is considered MEDICALLY NECESSARY.
- For individuals suspected of having ehrlichiosis and/or anaplasmosis (see Note 8), the use of an IFA assay for IgM antibodies or standard blood culture is considered NOT MEDICALLY NECESSARY.
- For individuals suspected of having malaria (see Note 10), the use of a rapid immunochromatographic diagnostic test or smear microscopy to diagnose malaria, determine the species of Plasmodium, identify the parasitic life-cycle stage, and/or quantify the parasitemia (can be repeated up to three times within three days if initial microscopy is negative in suspected cases of malaria) is considered MEDICALLY NECESSARY.
- To confirm the species of Plasmodium in an individual diagnosed with malaria, PCR testing is considered MEDICALLY NECESSARY.
- For individuals suspected of having malaria (see Note 10), the use of IFA for Plasmodium antibodies is considered NOT MEDICALLY NECESSARY.
- For individuals suspected of having a rickettsial disease (see Note 11), the use of an IFA assay for IgG antibodies (two tests occurring a minimum of two weeks apart) is considered MEDICALLY NECESSARY.
- For individuals suspected of having a rickettsial disease (see Note 11), the use of standard blood culture, NAAT, or IFA assay for IgM antibodies is considered NOT MEDICALLY NECESSARY.
- For individuals suspected of having West Nile virus (WNV) disease (see Note 12), the use of IFA for WNV-specific IgG or IgM antibodies in either serum or CSF and a confirmatory plaque reduction neutralization test for WNV is considered MEDICALLY NECESSARY.
- To confirm a WNV infection in individuals who are immunocompromised, nucleic acid detection of WNV is considered MEDICALLY NECESSARY.
- For immunocompetent individuals suspected of having WNV disease (see Note 12), the use of NAAT for WNV is considered NOT MEDICALLY NECESSARY.
- For individuals suspected of having a yellow fever virus (YFV) infection (see Note 13), the use of NAAT for YFV or serologic assays to detect virus-specific IgM and IgG antibodies, as well as a confirmatory plaque reduction neutralization test for YFV, is considered MEDICALLY NECESSARY.
- For the detection of Zika virus, the use of NAAT is considered MEDICALLY NECESSARY in the following individuals:
- Up to 12 weeks after the onset of symptoms for symptomatic (see Note 8) pregnant individuals who, during pregnancy, have either lived in or traveled to areas with current or past Zika transmission or who have had sex with someone who either lives in or has recently traveled to areas with current or past Zika virus transmission (see Note 14).
- For symptomatic non-pregnant individuals living in or with recent travel to an area with an active CDC Zika Travel Health Notice or an area with current or past Zika virus transmission (see Note 14) when symptoms presented within the last seven days.
- Zika virus NAAT and Zika virus IgM testing, as well as a confirmatory plaque reduction neutralization test for Zika, is considered MEDICALLY NECESSARY in any of the following situations:
- Up to 12 weeks after the onset of symptoms for symptomatic (see Note 8) pregnant individuals who, during pregnancy, have either lived in or traveled to areas with an active CDC Zika Travel Health Notice or who have had sex with someone who either lives in or has recently traveled to areas with an active CDC Zika Travel Health Notice (see Note 14).
- For pregnant individuals who have a fetus with prenatal ultrasound findings consistent with congenital Zika virus infection (see Note 15).
- For infants born from individuals who, during pregnancy, tested positive for Zika virus.
- For infants born with signs and symptoms of congenital Zika syndrome (see Note 15) and who have a birthing parent who had a possible Zika virus exposure during pregnancy.
- For symptomatic non-pregnant individuals living in or with recent travel to an area with an active CDC Zika Travel Health Notice or an area with current or past Zika virus transmission (see Note 14) when symptoms presented more than seven days prior to testing.
- For non-pregnant individuals who have not traveled outside of the United States and its territories and who are symptomatic for Zika virus infection (see Note 8), NAAT and/or IgM testing for Zika detection is considered NOT MEDICALLY NECESSARY.
- For asymptomatic individuals, testing for babesiosis, chikungunya virus, CTF, DENV, ehrlichiosis and/or anaplasmosis, malaria, rickettsial disease, TBRF, WNV, YFV, or Zika virus during a general exam without abnormal findings is considered NOT MEDICALLY NECESSARY.
NOTES:
Note 1: Typical signs and symptoms of babesiosis can include hemolytic anemia, splenomegaly, hepatomegaly, jaundice, and nonspecific flu-like symptoms such as fever, chills, body aches, weakness, and fatigue (CDC, 2024j).
Note 2: Typical signs and symptoms of HTRF (caused by Borrelia miyamotoi) can include chills or shakes, fatigue, nausea or vomiting, headache, and muscle and joint aches (CDC, 2024a).
Note 3: Typical signs and symptoms of LBRF (caused by Borrelia recurrentis) can include fever, headache, chills or shakes, muscle and joint aches, and nausea. Though the clinical symptoms of LBRF are similar to STRF, LBRF is usually associated with fewer relapses (CDC, 2024b)
Note 4: Typical signs and symptoms of STRF/TBRF (caused by Borrelia hermsii, B. turicatae, and other Borrelia bacteria) can include fever, headache, muscle aches, chills, dizziness, joint pain, nausea and vomiting, appetite loss, and rarely, facial paralysis eye pain or redness, or vision changes (CDC, 2024c).
Note 5: Typical signs and symptoms of chikungunya include high fever (> 102◦F or 39◦C), joint pains (usually multiple joints, bilateral, and symmetric), headache, myalgia, arthritis, conjunctivitis, nausea, vomiting, and maculopapular rash (Staples et al., 2024).
Note 6: Typical signs and symptoms of CTF can include fever, chills, headache, myalgia, malaise, sore throat, vomiting, abdominal pain, and maculopapular or petechial rash (CDC, 2024e).
Note 7: Typical signs and symptoms of dengue include fever, headache, retro-orbital eye pain, myalgia, arthralgia, macular or maculopapular rash, petechiae, ecchymosis, purpura, epistaxis, gingival bleeding, hematuria, leukopenia, thrombocytopenia, hyponatremia, elevated AST and ALT, and nausea and/or vomiting (CDC, 2024f, 2024r).
Note 8: Typical signs and symptoms of Zika virus infection can include fever, rash, headache, joint pain, conjunctivitis (red eyes), and muscle pain (CDC, 2024t).
Note 9: Typical signs and symptoms of ehrlichiosis and/or anaplasmosis usually begin 5 – 14 days after an infected tick bite, and they include fever, headache, malaise, myalgia, and shaking chills. Ehrlichiosis can also present with gastrointestinal issues, including nausea, vomiting, and diarrhea (Biggs et al., 2016).
Note 10: Typical signs and symptoms of malaria can include fever, influenza-like symptoms (e.g., chills, headache, body aches), anemia, jaundice, seizures, mental confusion, kidney failure, and acute respiratory distress syndrome (Tan & Abanyie, 2024).
Note 11: Typical signs and symptoms of rickettsial diseases (including Rocky Mountain spotted fever, Rickettsia parkeri rickettsiosis, Rickettsia species 364D rickettsiosis, Rickettsia spp. (mild spotted fever), and R. akari (rickettsialpox)) usually begin 3 – 12 days after initial bite and can include fever, headache, chills, malaise, myalgia, nausea, vomiting, abdominal pain, photophobia, anorexia, and skin rash. Rickettsia species 364d rickettsiosis can also present with an ulcerative lesion with regional lymphadenopathy (Biggs et al., 2016).
Note 12: Typical signs and symptoms of WNV include headache, myalgia, arthralgia, gastrointestinal symptoms, and maculopapular rash. Less than 1% of infected individuals develop neuroinvasive WNV with symptoms of meningitis, encephalitis, or acute flaccid paralysis (Nasci et al., 2013).
Note 13: Typical signs and symptoms of yellow fever include symptoms of the toxic form of the disease (jaundice, hemorrhagic symptoms, and multisystem organ failure), as well as nonspecific influenza symptoms (fever, chills, headache, backache, myalgia, prostration, nausea, and vomiting in initial illness) (Gershman & Staples, 2024).
Note 14: The CDC provides information on the geographic risk classifications of Zika (https://www.cdc.gov/zika/geo/index.html), as well as providing travel health notices for pathogens of concern (https://wwwnc.cdc.gov/travel/notices).
Note 15: Typical signs and symptoms of congenital Zika syndrome can include microcephaly, problems with brain development, feeding problems (e.g., difficulty swallowing), hearing loss, seizures, vision problems, decreased joint movement (i.e., contractures), and stiff muscles (making it difficult to move) (CDC, 2024n).
Table of Terminology
| Term |
Definition |
| AAP |
American Academy of Pediatrics |
| ASM |
American Society for Microbiology |
| CDC |
Centers for Disease Control and Prevention |
| CMS |
Centers for Medicare & Medicaid Services |
| CSF |
Cerebrospinal fluid |
| CTF/CTFV |
Colorado tick fever /virus |
| CV |
Coefficient of variation |
| DENV |
Dengue virus |
| DENV NS1 |
Dengue virus nonstructural protein 1 |
| DHF |
Dengue hemorrhagic fever |
| DNA |
Deoxyribonucleic acid |
| EDTA |
Ethylenediaminetetraacetic acid |
| EIA |
Enzyme immunoassay |
| ELISA |
Enzyme-linked immunosorbent assays |
| EM |
Erythema migrans |
| ESR |
Erythrocyte sedimentation rate |
| FDA |
Food and Drug Administration |
| FFPE |
Formalin-fixed, paraffin-embedded |
| FISH |
Fluorescent in situ hybridization |
| GlpQ |
Glycerophosphodiester phosphodiesterase gene |
| HAI |
Hemagglutination inhibition test |
| HTRF |
Hard tick relapsing fever |
| IDSA |
Infectious Diseases Society of America |
| IEC |
International Encephalitis Consortium |
| IFA |
Indirect immunofluorescence antibody |
| IFAs |
Immunofluorescence assays |
| IgG |
Immunoglobulin G |
| IgM |
Immunoglobulin M |
| IHC |
Immunohistochemistry |
| IMCA |
Immunochemiluminometric assay |
| LBRF |
Louse-borne relapsing fever |
| LDTs |
Laboratory developed tests |
| MAbs |
Monoclonal antibodies |
| MAC-ELISA |
IgM antibody capture enzyme-linked immunosorbent assay |
| MIA |
Microsphere-based immunoassay |
| MIF |
Microimmunofluorescent |
| NAAT |
Nucleic acid amplification testing |
| NDPH |
New daily persistent headache |
| NNDSS |
National Notifiable Disease Surveillance System |
| PCR |
Polymerase chain reaction |
| PRNT |
Plaque reduction neutralization test |
| PRNTs |
Plaque reduction neutralization tests |
| PT |
Prothrombin time |
| PTT |
Partial thromboplastin time |
| qPCR |
Quantitative polymerase chain reaction |
| RDT |
Rapid diagnostic testing |
| RMSF |
Rocky Mountain spotted fever |
| RNA |
Ribonucleic acid |
| RT-PCR |
Real-time polymerase chain reaction |
| SFG |
Spotted fever group |
| STRF |
Soft tick relapsing fever |
| TBRF |
Tickborne relapsing fever |
| WHO |
World Health Organization |
| WNV |
West Nile virus |
| YFV |
Yellow fever virus |
Rationale
Hematophagous arthropods, such as mosquitoes, ticks, fleas, and mites, can spread opportunistic bacteria, protozoa, and viruses to host organisms when feeding. Numerous outbreaks of arthropod-borne disease have been documented, including plague, an acute febrile disease caused by Yersinia pestis through the bite of infected fleas, which resulted in more than 50 million deaths in Europe alone during the “Black Death” outbreak. More than 3000 cases of plague were reported to the World Health Organization (WHO) between 2010 and 2015 with 584 deaths. Today, most cases of plague occur in the Democratic Republic of Congo, Madagascar, and Peru (WHO, 2022b).
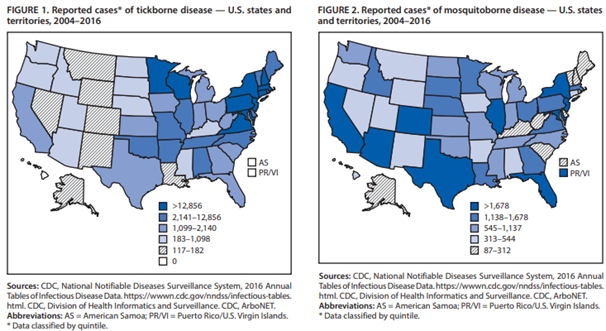
The Centers for Disease Control and Prevention (CDC) reported a large increase in the number of vector-borne diseases within the United States and its territories between 2004-2016. More than 640,000 cases were reported during that time; in fact, infections of tickborne bacteria and protozoa more than doubled from 2004 to 2016. “In the United States, 16 vector-borne diseases are reportable to state and territorial health departments, which are encouraged to report them to the National Notifiable Disease Surveillance System (NNDSS). Among the diseases on the list that are caused by indigenous pathogens are Lyme disease (Borrelia burgdorferi); West Nile, dengue, and Zika virus diseases; plague (Yersinia pestis); and spotted fever rickettsioses (e.g., Rickettsia rickettsii). Malaria and yellow fever are no longer transmitted in the United States but have the potential to be reintroduced” (Rosenberg et al., 2018). New vector-borne infections are emerging; for example, two unknown, life-threatening RNA viruses spread by ticks have been identified in the U.S. since 2004. Although both tick- and mosquito-borne diseases are increasing across the U.S., the CDC reports that these two vectors are showing different trends. The mosquito-borne diseases are characterized by epidemics; for example, West Nile Virus is essentially limited to the continental U.S. but has spread rapidly since its introduction to New York in 1999, whereas chikungunya and dengue primarily occur within the U.S. territories. On the other hand, the tickborne disease increase occurs in the continental U.S. and has experienced a gradual, steady rate increase with Lyme disease comprising 82% of all tickborne diseases (Rosenberg et al., 2018). Figure 1 and 2 below, taken from Rosenberg et al. (2018), show the reported cases of tickborne and mosquito-borne disease in the United States from 2004-2016.
Rickettsial infections
Rocky Mountain spotted fever (RMSF) is the most common rickettsial infection in the U.S. with 6,248 cases reported to the CDC alone in 2017 (CDC, 2024q). RMSF is caused by Rickettsia rickettsii, spread in the U.S. predominantly by Dermacentor variabilis (the American dog tick) and D. andersoni (the Rocky Mountain wood tick), and can be found throughout North America as well as parts of South America. The Council for State and Territorial Epidemiologists combined RMSF with other rickettsial diseases into the more broad “spotted fever rickettsiosis” designation in 2010 (CDC, 2024q). Besides the obligatory tick bite, typical symptoms of RMSF include fever, headache, and rash with the characteristic rash occurring in approximately 88% to 90% of patients within three to five days of illness. If left untreated, RMSF can be fatal but can easily be treated with antimicrobial therapy upon timely diagnosis. Definitive diagnosis of RMSF cannot usually be made via culture because Rickettsia cannot be grown in cell-free culture media; they are obligate intracellular bacteria requiring living host cells. RMSF diagnosis can be made via either skin biopsy prior to treatment with antibiotics or through serologic testing using IFAs. Immunoglobulin G (Biggs et al.) antibodies are more specific than immunoglobulin M (IgM) antibodies since the latter can give false-positive results due to cross-reactivity with other bacterial pathogens. A drawback of IFA is that usually it is unreliable for the first five days of infection until antibody levels are high enough for detection. The CDC and major clinical labs do offer a polymerase chain reaction (PCR)-based assay for RMSF (McClain, 2024a).
Since 2001, thirteen more human Rickettsiae belonging to the spotted fever group (SFG) have been identified. All SFGs can cause fever, headache, and myalgia and are arthropod-borne (primarily ticks and mites). Most patients with an SFG display a rash and/or a localized eschar. Rickettsialpox, caused by R. akari, is transmitted from the bite of a house mouse mite, usually after mouse extermination programs result in a decrease of the mite’s food supply. Rickettsialpox is typically a relatively mild disease that can resolve itself without treatment within three weeks, but treatment hastens improvement. Rickettsiosis can also be due to infection with R. parkeri, R. amblyommii, and Rickettsia species 364D (also called R. philipii). Isolation of SFG Rickettsiae is rare in clinical practice due to the difficulty of obtaining culture; consequently, serology, immunologic detection from tissue, and PCR are more often used for diagnosis. Microimmunofluorescent (MIF) antibody tests, enzyme-linked immunosorbent assays (ELISAs), and Western blot immunoassays can be used to detect convalescent IgG and IgM antibodies, but these methods can only be used at least 10-14 days after the onset of illness when antibody concentrations are high enough for detection. McQuiston et al. (2014) concluded that the “use of IgM antibodies should be reconsidered as a basis for diagnosis and public health reporting of RMSF and other spotted fever group rickettsia in the United States” in one small study; the study demonstrated that IgM findings often resulted in false positives for Rock Mountain Spotted Fever and questioned the value of IgM testing (McQuiston et al., 2014). PCR is a very specific technique. PCR using tissue samples has higher specificity than whole blood PCR. Immunologic detection from a tissue biopsy requires the use of special laboratory equipment so it is not as frequently used as either the serologic or PCR detection methods (McClain, 2024c).
Ehrlichiosis and Anaplasmosis
Human ehrlichiosis was first reported in 1986, and the causative agent for human granulocytic anaplasmosis, Anaplasma phagocytophilum, was identified in 1994. Both ehrlichiosis and anaplasmosis are transmitted from the bite of infected ticks and have similar clinical and laboratory manifestations. Ehrlichiosis can be caused by Ehrlichia chaffeensis, E. ewingii, and E. muris. Typically, patients have a fever within an incubation period of one to two weeks. Other symptoms can include malaise, myalgia, headache, chills, gastrointestinal distress, and cough. Both leukopenia and thrombocytopenia can occur. Diagnosis via culture is extremely difficult. “Until 1995, only two isolates of E. chaffeensis had been recovered from humans; in both cases, this process required over 30 days of cultivation. The isolation of A. phagocytophilum from three additional patients has been accomplished using a cell culture system derived from human promyelocytic leukemia cells (McClain, 2024b). IFA testing for bacteria-specific antibodies is the most common method for diagnosing ehrlichiosis and anaplasmosis, but similar to rickettsiae, ELISA, PCR, and immunochemical tissue staining can be used as well. Unlike rickettsiosis, ehrlichiosis and anaplasmosis can also be detected by the presence of characteristic intraleukocytic morulae in a peripheral blood smear or buffy coat smear (McClain, 2024b).
Borrelia Infections
Besides Lyme disease, caused by Borrelia burgdorferi, Borrelia can cause relapsing fever. Tick-borne relapsing fever (TBRF) in North America is primarily caused by B. hermsii, B. turicatae, B. parkeri, B. miyamotoi, and B. mazzottii, and louse-borne relapsing fever (LBRF) is an infection caused by B. recurrentis (Barbour, 2024; Miller et al., 2024). The characteristic feature of these infections is the relapsing fever due to cyclical spirochetemia caused by antigenic variation of the spirochetes. Each bout of fever lasts three to 12 days with temperatures ranged from 39◦C to 43◦C (102.2◦F to 109.4◦F). Visual analysis by Giemsa or Wright staining blood smears taken during a febrile episode is common practice. PCR can also be used on a variety of samples, including cerebrospinal fluid (CSF), blood, tissue, or even culture medium. According to the CDC, “a change in serology results from negative to positive, or the development of an IgG response in the convalescent sample, is supportive of a TBRF diagnosis” (CDC, 2024p). One exception is using antibodies to the GlpQ protein characteristic of these Borrelia species but not to B. burgdorferi (Lyme disease) (Barbour, 2024).
Protozoa infections
Babesiosis is due to primarily Babesia microti in the U.S, but B. divergens and B. venatorum are the primary causative agents of babesiosis in Europe and China, respectively. The incubation period of Babesia depends on the mode of transfection: one to four weeks following a tick bite; the incubation period after transfusion of contaminated blood products usually or three to seven weeks but ranges from one week to six months. The most common symptoms of infection include a fever, fatigue, malaise, chills, sweats, headache, and myalgia. Immunocompromised individuals can develop relapsing babesiosis due to an absent or impaired production of antibodies with approximately 20% mortality rate for patients who develop relapsing babesiosis. Most patients with babesiosis are also co-infected with other tickborne bacterial pathogens. “Preferred tools for diagnosis of babesiosis include blood smear for identification of Babesia organisms and polymerase chain reaction (PCR) for detection of Babesia DNA. Serology can be a useful adjunct to blood smear and PCR” (Krause & Vannier, 2024). Serology is not ideal in diagnosing an acute infection since antibody concentrations remain elevated post-recovery.
Plasmodium falciparum, P. vivax, and P. ovale are responsible for malaria. They are spread by the bite of an Anopheles mosquito where their sporozoites infect the liver within one to two hours. Within the hepatocyte, they form merozoites. Upon rupturing into the bloodstream, the merozoites infect red blood cells for trophozoite formation, causing the erythrocytic stage of the life-cycle where additional merozoites are released. During this stage of the cycle, the symptoms of malaria, including fever, occur. This process usually takes 12 to 35 days, but clinical manifestations can be delayed in individuals with partial immunity or those who are taking ineffective prophylaxis. Other initial symptoms can include irregular heartbeat, cough, anorexia, gastrointestinal distress, sweating, chills, malaise, arthralgia, and myalgia. Malaria, if left untreated, can also include acidosis, hypoglycemia, severe anemia, renal and hepatic impairment, edema, and death (Cohee & Seydel, 2022). Parasite-based diagnosis may include microscopic examination of blood smears, which can often identify the species of Plasmodium as well as the parasite density, and antigen-based tests. Rapid diagnostic testing (RDT) of the antigens using immunochromatographic methods is available, but the accuracy of the RDT can vary considerably. NAATs can also be used to identify a malarial infection, and NAATs “are typically used as a gold standard in efficacy studies for antimalarial drugs, vaccines, and evaluation of other diagnostic agents” with a “theoretical limit of detection for PCR … estimated at 0.02 to 1 parasite/microL” (Hopkins, 2023). The Mayo Clinic Laboratories indicates that “PCR is an alternative method of malaria diagnosis that allows for sensitive and specific detection of Plasmodium species DNA from peripheral blood. PCR may be more sensitive than conventional microscopy in very low parasitemias, and is more specific for species identification…Malaria PCR can be used in conjunction with traditional blood film or Babesia PCR when the clinical or morphologic differential includes both babesiosis and malaria” Clinic (2024).
Viral infections
Examples of arthropod-borne viruses (arboviruses) include West Nile virus (WNV), dengue, yellow fever virus (YFV), chikungunya, and Colorado tick fever virus. In the United States, WNV is the most common arbovirus reported to the CDC. In 2016, 96% of the reported 2,240 cases of domestic arboviruses were WNV with 61% of the WNV cases reported being neuroinvasive. Neuroinvasive WNV includes meningitis, encephalitis, and acute flaccid paralysis (Burakoff et al., 2018). In general, most infected individuals are asymptomatic with only 20% – 40% of infected patients showing any characteristic symptoms of WNV, including fever, headache, malaise, myalgia, anorexia, and rash. Diagnosis of WNV of a symptomatic individual usually occurs with a WNV IgM antibody capture ELISA (MAC-ELISA) assay. A patient with symptoms of a neurologic infection does require a lumbar puncture. Confirmatory testing can include a plaque reduction neutralization test (PRNT). PCR testing is primarily used with immunocompromised patients who have delayed or absent antibody production, patients with a history of prior flavivirus infections, and blood donors who may be asymptomatic (Petersen, 2022).
Dengue virus (DENV) infection is a result of being bitten by an infected Aedes aegypti or A. albopictus mosquito. Four distinct DENV types of Flavivirus are known: DENV-1, DENV-2, DENV-3, and DENV-4. DENV is endemic throughout much of the tropical regions of the world, but the only region of the U.S. endemic for DENV is Puerto Rico. The last major outbreak occurred in Puerto Rico in 2010 where 26,766 cases of suspected DENV were reported and 47% of all laboratory tested specimen were positive (CDC, 2024f). “Dengue fever … is an acute febrile illness defined by the presence of fever and two or more of the following but not meeting the case definition of dengue hemorrhagic fever: headache, retro-orbital or ocular pain, myalgia and/or bone pain, arthralgia, rash, hemorrhagic manifestations … [and] leukopenia. The cardinal feature of dengue hemorrhagic fever is plasma leakage due to increased vascular permeability as evidenced by hemoconcentration (≥ 20 percent rise in hematocrit above baseline), pleural effusion, or ascites. DHF [dengue hemorrhagic fever] is also characterized by fever, thrombocytopenia, and hemorrhagic manifestations ….” (Thomas et al., 2022). Laboratory diagnostic testing includes direct detection of viral components in serum or indirect serologic assays. “Detection of viral nucleic acid or viral antigen has high specificity but is more labor intensive and costly; serology has lower specificity but is more accessible and less costly” (Thomas et al., 2022). Culture testing as a diagnostic tool usually is time-prohibitive.
Zika virus is a mosquito-borne illness discovered in Uganda in 1947 but has since spread across Asia and to the Americas. Zika infection has been tied to several birth defects. The first human cases of Zika were detected in 1952. Prior to 2007, at least 14 cases of Zika had been documented. Symptoms of Zika are similar to those of many other diseases; therefore, many cases may not have been recognized (CDC, 2024t). The most common symptoms of Zika are fever, rash, joint pain, and conjunctivitis (CDC, 2024t). The illness is usually mild with symptoms beginning two to seven days after being bitten by an infected mosquito, lasting for several days to a week. Most individuals infected with Zika virus are unaware of the infection, as only a maximum of 25% of people infected will exhibit symptoms (CDC, 2024t; LeBeaud, 2023). Diagnosis of the Zika virus is definitively established through reverse-transcription polymerase chain reaction (RT-PCR) for Zika virus RNA in all symptomatic patients. Aside from pregnant individuals who have traveled to an at risk area, asymptomatic patients are typically not tested (LeBeaud, 2023).
Colorado tick fever virus (CTFV) is a Reoviridae transmitted primarily by the Rocky Mountain wood tick (Dermacentor andersoni) in the western U.S. and Canada. Transmission of CTFV has also been reported in blood transfusions. The incubation period can last up to 14 days, and symptoms include fever, headache, chills, myalgia, leukopenia, and prostration. Only 15% of symptomatic patients demonstrate a rash. Serologic tests are usually not helpful until at least 10-14 days for antibody production whereas real-time PCR (RT-PCR) can be used on the first day of symptoms (Petersen, 2021).
Yellow fever, occurring primarily in sub-Saharan Africa and South America, is a flavivirus spread by mosquitoes that causes hemorrhagic fever with a high fatality rate. An outbreak in Brazil in January-March 2018 resulted in four of ten patients infected with YFV dying. None of those showing symptoms had been vaccinated against YFV. Yellow fever causes hemorrhagic diathesis due to decreased synthesis of vitamin K-dependent coagulation factors as well as hepatic dysfunction, renal failure, and coagulopathy. Yellow fever diagnosis is typically made by a serologic test using an ELISA-IgM assay; however, this assay does cross-react with other flaviviruses and with the YFV vaccination. Rapid diagnostic testing using either PCR or immunoassay is available. Viral isolation and culture can be performed, but it requires inoculation of mosquitoes or mammalian cell culture. Tissue biopsy, such as liver, cannot be performed on the living patient due to possible fatal hemorrhaging; biopsy would be performed during the post-mortem workup (Wilder-Smith, 2024).
Chikungunya virus, endemic in many tropical and subtropical regions of the world, is transmitted by the mosquitoes Aedes aegypti and Aedes albopictus. Within the U.S., chikungunya is prevalent in Puerto Rico where approximately 25% of blood donors were seropositive; it has also been reported in Florida. Both dengue and Zika are transmitted by the same vectors, so these viruses often co-circulate geographically Chikungunya can cause acute febrile polyarthralgia and arthritis. The predominant testing method for diagnosis of chikungunya is the detection of viral RNA via either RT-PCR or virus serology using either ELISA or IFA. Viral culture is typically not used as a diagnostic tool but is used for epidemiologic research (Wilson & Lenschow, 2022).
Types of Testing
| Test |
Description |
Rationale |
| Culture |
Culture growth depends on the pathogen being studied. If the pathogen is an obligate intracellular organism, then it must be isolated using more sophisticated cell culture techniques. In many circumstances, culture is used for research and/or epidemiology rather than as a diagnostic tool (Biggs et al., 2016; Miller et al., 2024). |
At times, culture testing is not as sensitive as either NAAT or serologic testing and can be time-intensive when treatment should not be delayed. Depending on the organism, this may require high biosafety level laboratory for culture growth (Biggs et al., 2016). |
| Indirect immunofluorescene antibody (IFA) assays |
IFA is a serologic assay that can be used to test for the presence of antibodies, such as IgG and IgM, reactive against the pathogen (Biggs et al., 2016). |
Depending on the pathogen, IFA can be a useful tool. At times, though, it can cross-react with either a prior vaccination or infection (Wilder-Smith, 2024). An acute infection can often be determined by performing IFA in both the acute phase and convalescent phase where at least a fourfold increase in antibodies is indicative of an acute infection (Biggs et al., 2016). |
| Darkfield microscopy |
Darkfield microscopy can be used to detect the presence of microorganisms, such as motile spirochetes (Miller et al., 2024). |
This technique is not widely available, and transport of sample must be done immediately if testing of motile specimen is desired (Miller et al., 2024). |
| Blood-smear microscopy |
Blood-smear microscopy can be either thick or thin and is typically performed on a sample stained with an eosin-azure-type dye, such as Giemsa, to look at intracellular structures or morphological features (Biggs et al., 2016). |
This technique should be performed by an experienced microscopist since it can be inconsistent. As compared to other techniques, this technique is relatively inexpensive (Biggs et al., 2016). |
| Nucleic acid amplification testing (NAAT) |
NAATs can include polymerase chain reaction (PCR), real-time PCR (RT-PCR), or other enzyme-dependent amplification testing for the presence of nucleic acids (DNA or RNA). |
NAATs can be specific and sensitive; however, they may not be available at all laboratories and/or can be costly. Some NAATs are available as rapid diagnostic tools. NAATs have been used on serum, whole blood, tissue, CSF, and even formalin-fixed, paraffin-embedded biopsies from autopsy tissues. The sensitivity of the technique can vary depending on the sample; for example, whole blood PCR for R. rickettsii is less sensitive than a similar sample test for E. chaffeensis (Biggs et al., 2016). |
Analytical Validity
The use of antibodies to detect and diagnose arthropod-associated infections and diseases is a common practice. Johnson et al. (2000) first reported the use of monoclonal antibody-based capture ELISA testing for a variety of alphaviruses, including chikungunya, flaviviruses, including dengue and yellow fever, and bunyaviruses. The researchers concluded, “IgG ELISA results correlated with those of the standard plaque-reduction neutralization assays. As expected, some test cross-reactivity was encountered within the individual genera, and tests were interpreted within the context of these reactions. The tests were standardized for laboratory diagnosis of arboviral infections, with the intent that they be used in tandem with the corresponding IgM antibody-capture ELISAs” (Johnson et al., 2000). Kalish et al. (2001) also demonstrated that IgG and/or IgM antibody responses can still occur up to 20 years post-infection; consequently, a rise in antibody titer does not necessarily indicate a current, acute infection (Kalish et al., 2001).
Granger and Theel (2019) published an evaluation of two enzyme-linked immunosorbent assays and a rapid immunochromatographic assay for the detection of IgM antibodies to Zika virus. This article states that five serological assays have been approved by the FDA in an emergency use situation and include the Chembio DPP Zika IgM system (a rapid immunochromatographic assay), the InBios ZIKV Detect 2.0 IgM antibody capture enzyme-linked immunosorbent assay, and the InBios ZIKV Detect MAC-ELISA. These three serologic assays were evaluated, using 72 samples, based on the identification of neutralizing antibodies to Zika virus, dengue virus, or West Nile virus. “The Chembio DPP Zika ICA and InBios ZIKV 2.0 MAC-ELISA showed 95% specificity in 22 ZIKV/DENV-seronegative specimens and in 13 samples positive for NAbs to non-ZIKV flaviviruses. Comparatively, the InBios ZIKV MAC-ELISA was “presumptive” or “possible Zika positive” in 8 of 12 WNV or DENV PRNT-positive samples and in 12 of 22 PRNT-seronegative sera” (Granger & Theel, 2019). The authors conclude that by replacing the InBios ZIKV MAC-ELISA with the InBios ZIKV 2.0 MAC-ELISA, testing burden will be minimized on laboratories performing PRNT for the identification of neutralizing antibodies.
Leski et al. (2020) performed a 2020 study published in the Malaria Journal that compared traditional diagnostic methods such as rapid diagnostic tests (RDTs) and DNA-based methods to polymerase chain reaction (PCR). The results indicated consistency with “previous observations that PCR-based tests have a significantly higher sensitivity when compared with both microscopy and RDTs” (Leski et al., 2020).
Mathison and Pritt (2017) reviewed current standards for malaria testing and the most used methods for laboratory diagnosis. The most common tests “are microscopic examination of stained blood films and detection of parasite antigen or nucleic acid. … Rapid antigen detection methods and molecular amplification tests are also increasingly employed for malaria diagnosis and are useful adjunctive tests.” According to the algorithm developed in “Update on Malaria Diagnostics and Test Utilization,” NAAT tests are one of three tests recommended for use if malaria is suspected based on clinical findings and exposure history (Mathison & Pritt, 2017).
Kim et al. (2018) had also developed a rapid diagnostic test (RDT) for detecting IgG/IgM antibodies against Zika virus using “monoclonal antibodies to the envelope (E) and non-structural protein (NS1).” The diagnostic accuracy of this kit was “fairly high; sensitivity and specificity for IgG was 99.0 and 99.3%, respectively, while for IgM it was 96.7% and 98.7%, respectively.” However, there were cross reactions with the dengue virus evaluated using anti-Dengue Mixed Titer Performance Panel (PVD201), “in which the Zika RDT showed cross-reactions with [dengue virus] in 16.7% and 5.6% in IgG and IgM, respectively.” This research could potentially enable the rapid diagnostic test to be preferable to the traditional RT-PCR in endemic areas (Kim et al., 2018).
Clinical Utility and Validity
Kato et al. (2013) tested the sensitivity of two different RT-PCR-based assays for Rickettsia — PanR8, an assay that tests for Rickettsia in general, and RRi6, an assay specific for R. rickettsii. Both of these methods were more sensitive in testing for Rickettsia than the nested PCR method of the CDC; moreover, both of these methods are faster than the nested PCR method (one hour versus one to two days, respectively) (Kato et al., 2013). These results were corroborated in 2014 by Denison and colleagues. They used a multiplex PCR assay to correctly identify all cell controls for R. rickettsii, R. parkeri, and R. akari; moreover, no false-positive results were reported using this methodology. “This multiplex real-time PCR demonstrates greater sensitivity than nested PCR assays in FFPE [formalin-fixed, paraffin-embedded] tissues and provides an effective method to specifically identify cases of Rocky Mountain spotted fever, rickettsialpox, and R. parkeri rickettsiosis by using skin biopsy specimens” (Denison et al., 2014).
The FDA has approved the use of the BinaxNOW malaria test for screening and diagnosing malaria. Even though this testing method is considerably faster than other methods (as low as 1.1 – 1.7 hours complete turnaround time (Ota-Sullivan & Blecker-Shelly, 2013), the use of BinaxNOW in non-endemic areas is a point of controversy due to relatively low sensitivity (84.2%) and for misclassifying Plasmodium falciparum malaria as non-falciparum (Dimaio et al., 2012). Moreover, it has been reported that Salmonella typhi can give a false-positive for malaria using the BinaxNOW test (Meatherall et al., 2014).
van Bergen et al. (2021) evaluated a novel real-time PCR assay for clinical validity. The authors used reference samples, patient samples, and synthetic controls. The analytical performance details of the MC004 assay were considered: “analytical specificity, limit of detection, the ability to detect mixed infections, and the potential to determine the level of parasitaemia of P. falciparum, including assessment of within-run and between-run precisions.” The authors reported “zero false positive or false negative results.” Regarding precision, “the within-run and between-run precisions were less than 20% CV at the tested parasitaemia levels of 0.09%, 0.16%, 2.15% and 27.27%.” Based on these results, the authors reported that “the entry of PCR-based techniques into malaria diagnostics has improved the sensitivity and specificity of the detection of Plasmodium infections. … Based upon the analytical performance characteristics that were determined, the MC004 assay showed performance suitable for use in clinical settings, as well as epidemiological studies” (van Bergen et al., 2021).
Akoolo et al. (2017) compared qPCR results in the detection of Babesia infection against currently available non-NAAT tests (FISH and microscopy). Blood samples were analyzed from 192 patients. The researchers report that “Of 28 samples that were positive by FISH, 27 (96%) were also positive by qPCR indicating high congruency between nucleic acid-based tests. Interestingly, of 78 asymptomatic samples not tested by FISH, 22 were positive by our qPCR” (Akoolo et al., 2017). Overall, the qPCR method was found to have a sensitivity of 96.2% and a specificity of 70.5%. The authors conclude, “Robust qPCR using specific probes can be highly useful for efficient and appropriate diagnosis of babesiosis in patients in conjunction with conventional diagnostics, or as a stand-alone test, especially for donated blood screening” (Akoolo et al., 2017).
Reynolds et al. (2017) examined the 2016 United States Pregnancy Registry to estimate the proportion of birth defects of pregnant women exposed to Zika, and out of 972 pregnancies with laboratory evidence of a possible Zika infection, 51 had birth defects (five percent). Of the 250 confirmed infections, 24 had birth defects. Similarly, Shiu et al. (2018) evaluated the screening results of the Zika virus in Miami-Dade County in Florida. Of 2327 women screened for Zika, 86 had laboratory evidence of infection, and two had congenital Zika “syndrome” (Zika-caused birth defects) (Shiu et al., 2018).
Centers for Disease Control and Prevention (CDC)
Diagnosis and Management of Tickborne Rickettsial Diseases (Biggs et al., 2016): In 2016, the CDC released their guidelines and recommendations concerning Rickettsial diseases, including Rocky Mountain spotted fever, in the Morbidity and Mortality Weekly Report. The table below summarizes their recommended diagnostic tests for tickborne rickettsial diseases:
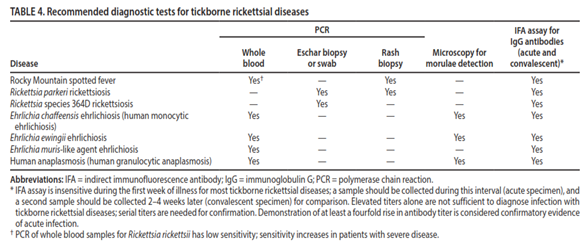
To summarize their recommendations, even though indirect immunofluorescence antibody assays (IFAs) are insensitive typically during the first week of an acute infection, they are the standard reference for tickborne rickettsial infections; in addition, a minimum of two tests are to be performed for a diagnosis. Usually, one sample is taken early after the initial symptoms are present, and a second sample is taken two to four weeks later. A minimum of a fourfold rise in antibody titer is required to confirm diagnosis. In cases of ehrlichiosis and anaplasmosis, during the first week, PCR amplification can be used on whole blood for diagnosis, but PCR has low sensitivity in Rocky Mountain spotted fever except in patients with severe disease. Morulae detection via either blood smear or buffy coat preparation microscopy can also be indicative of ehrlichiosis or anaplasmosis. However, “Rickettsiae cannot be isolated with standard blood culture techniques because they are obligate intracellular pathogens; specialized cell culture methods are required. Because of limitations in availability and facilities, culture is not often used as a routine confirmatory diagnostic method for tickborne rickettsial diseases” (Biggs et al., 2016).
In 2024, the CDC published updated guidelines pertaining to rickettsial infections, which provide similar guidelines to those published in 2016. “The standard serologic test for diagnosis of RMSF is the indirect fluorescent antibody (IFA) test for immunoglobulin G (IgG) using R. rickettsii antigen. IgG IFA assays should be performed on paired acute and convalescent serum samples collected 2–10 weeks apart to demonstrate evidence of a fourfold seroconversion. ... Single or inappropriately timed serologic tests, in relation to clinical illness, can lead to misinterpretation of results” (CDC, 2024d). They also provide statements on nucleic acid testing and IHC/culture testing for rickettsial infections: “PCR amplification is performed on DNA extracted from whole blood serum, or plasma. R. rickettsii infect the endothelial cells that line blood vessels and may not circulate in large numbers in the blood until the disease has progressed to a severe phase of infection. Although a positive PCR result is helpful, a negative result does not rule out the diagnosis, and treatment should not be withheld due to a negative result. PCR might also be used to amplify DNA from a skin biopsy of a rash lesion, or in post-mortem tissue specimens. . . Culture and IHC assays can also be performed on skin biopsies of a rash lesion, or post-mortem tissue specimens. Culture isolation and IHC assays of R. rickettsii are only available at specialized laboratories; routine hospital blood cultures cannot detect the organism” (CDC, 2024d).
Soft tick relapsing fever (STRF) /Tickborne relapsing fever (TBRF) (CDC, 2024c, 2024i): In the U.S., STRF/TBRF can be caused by Borrelia hermsii, B. turicatae, and other Borrelia bacteria via the bite of soft-bodied Ornithodoros genus ticks. STRF often presents with a relapsing nature, with symptoms appearing 4 – 21 days after exposure, with intermittent fevers lasting for three days and remitting for seven days before relapse. Moreover, “Spirochetes may be present in high concentrations in the blood of febrile patients (> 106 spirochetes/ml). Spirochetes are most readily detected by microscopy in symptomatic, untreated patients early in the course of infection. Direct visualization by microscopy using dark field or stained peripheral blood smears is generally adequate to confirm the diagnosis. … PCR is more sensitive than microscopy and may also be used during asymptomatic periods or soon after treatment initiation. The preferred specimen type for PCR testing is whole blood. … Serologic testing is available from some labs to diagnose STRF. Serologic assay results are most sensitive when specimens are collected at least 14 days after symptom onset. … Patients with relapsing fevers might have false positive serologic tests for Lyme disease” (CDC, 2024c).
The CDC acknowledges that some PCR and serologic tests may cross-react with other Borrelia species; thus, “clinical and epidemiologic features, such as travel and exposure history, are important to guide interpretation of test results. Consider a diagnosis of STRF for patients with positive Lyme disease or [hard tickborne relapsing fever] serology who have not been in areas endemic for these diseases.” Additionally, patients may exhibit other general laboratory findings, such as “thrombocytopenia, increased white blood cell count, mildly increased serum bilirubin level, elevated erythrocyte sedimentation rate (ESR), and slightly prolonged prothrombin time (PT) and partial thromboplastin time (PTT) (CDC, 2024i).
Hard tick relapsing fever (HTRF) (CDC, 2024a, 2024g): In the U.S., HTRF is used to differentiate between infections caused by hard-bodied ticks and soft-bodied ticks (see STRF above). HTRF is caused by the Borrelia miyamotoi bacteria and is transmitted through the bites of infected blacklegged ticks (Ixodes scapularis) and western blacklegged ticks (Ixodes pacificus). Unlike STRF, it causes a single episode of fever more commonly, with 10% of cases having a relapsing fever. Symptoms appear about two weeks after a tick bite but can occur within three to six days after exposure. Diagnosis is often made by PCR using whole blood, but several PCR and serologic methods cannot distinguish between HTRF and STRF. The CDC also adds “Serologic testing is available from some labs for diagnoses of HTRF. Serologic assay results are most sensitive when specimens are collected at least 14 days after symptom onset. Serum taken early during infection may yield negative results.” Similar emphasis is placed on considering clinical and epidemiological features when interpreting results, as HTRF patients may also test positive for other Borrelia species, such as Lyme disease (CDC, 2024a, 2024g).
Louse-borne relapsing fever (LBRF) (CDC, 2024b, 2024h): In the U.S., LBRF is caused by Borrelia recurrentis bacteria and transmitted by the human body louse, and rarely, head louse. It also occurs endemically in regions of Africa and in overcrowded conditions. Clinically, LBRF presents similarly to STRF but with fewer relapses. Diagnosis is made with “direct visualization of spirochetes in a peripheral blood smear in symptomatic, untreated patients early in the course of infection,” as “people with LBRF experience high levels of spirochetemia during febrile episodes.” Alternatives for diagnosis also include PCR, but the same precautions hold for LBRF as for HTRF and STRF when interpreting results (CDC, 2024b, 2024h).
Colorado Tick Fever (CTF) (CDC, 2024e): As of 2023, CTF was reportable in Arizona, Colorado, Idaho, Montana, New Mexico, Oregon, South Dakota, Utah, Washington, and Wyoming. “Laboratory diagnosis of CTF is generally accomplished by testing of serum to detect viral RNA or virus-specific immunoglobulin (Ig) M and neutralizing antibodies. Antibody production can be delayed with CTF, so tests that measure antibodies may not be positive for 14 – 21 days after the onset of symptoms. RT-PCR (reverse-transcriptase polymerase chain reaction) is a more sensitive test early in the course of disease. CTF testing is available at some commercial and state health department laboratories and at CDC. Contact your state or local health department for assistance with diagnostic testing. They can help you determine if samples should be sent to the CDC Arbovirus Diagnostic Laboratory for further testing” (CDC, 2024e).
Babesiosis (CDC, 2024j): Babesiosis is caused most commonly by Babesia microti, which is usually transmitted by white-footed mice and other small mammals. Diagnosis can be challenging due to the nonspecific clinical manifestations of the disease. “For acutely ill patients, the findings on routine laboratory testing frequently include hemolytic anemia and thrombocytopenia. Additional findings may include proteinuria, hemoglobinuria, and elevated levels of liver enzymes, blood urea nitrogen, and creatinine. When considering a babesiosis diagnosis, healthcare providers should explicitly request a manual (non-automated) review of the peripheral blood smear. In symptomatic patients with acute infection, it is typical to detect Babesia parasites through light-microscopic examination of blood smears, though multiple smears may need to be examined. Distinguishing between Babesia and Plasmodium (especially P. falciparum) parasites and artifacts like stain or platelet debris can be challenging. Consider having a reference laboratory confirm the diagnosis — by blood-smear examination and, if indicated, by other means, such as molecular and/or serologic methods tailored to the setting/species” (CDC, 2024j).
Malaria (Tan & Abanyie, 2024): The CDC considers smear microscopy as the gold standard in diagnosing malaria since it can determine the species, identify the stage of parasitic life-cycle, and quantify the parasitemia. The CDC states, “Blood smear microscopy remains the most important method for malaria diagnosis. Microscopy can provide immediate information about the presence of parasites, allow quantification of the density of the infection, and allow determination of the species of the malaria parasite — all of which are necessary for providing the most appropriate treatment. Tests should be performed immediately when ordered by a health care provider, and microscopy results should be available as soon as possible, ≤ 24 hours of the patient’s presentation. They should not be saved for the most qualified staff to perform or batched for convenience. In addition, these tests should not be sent out to reference laboratories with results available only days to weeks later. Assistance with speciation of malaria on smears is available from CDC” (Tan & Abanyie, 2024). The CDC also notes that rapid diagnostic tests (RDTs) for malaria can detect malaria parasitic antigens. However, “RDTs offer a useful alternative to microscopy in situations where reliable microscopic diagnosis is not immediately available. Although RDTs can detect malaria antigens within minutes, they have several limitations. RDTs cannot distinguish between all of the Plasmodium species that affect humans, they may be less sensitive than expert microscopy or PCR for diagnosis, they cannot quantify parasitemia, and an RDT-positive test result may persist for days or weeks after an infection has been treated and cleared. Thus, RDTs are not useful for assessing response to therapy. Furthermore, in some areas, mutations are increasingly being observed in malaria parasites, resulting in an absence of the malaria antigen usually detected by many RDTs, including the only RDT used in the United States. The absence of this parasite antigen in peripheral blood can lead to false-negative RDT test results. Both positive and negative RDT results must always be confirmed by microscopy. Microscopy confirmation of the RDT result should occur as soon as possible, because the information on the presence, density, and parasite species is critical for optimal management of malaria” (Tan & Abanyie, 2024). Regarding PCR, the CDC states that “These tests are more sensitive than routine microscopy, but results are not usually available as quickly as microscopy results, thus limiting the utility of this test for acute diagnosis and initial clinical management. Use of PCR testing is encouraged to confirm the species of malaria parasite and detect mixed infections” (Tan & Abanyie, 2024).
While diagnosis from microscopic examination remains the gold standard for laboratory confirmation of malaria, the CDC does acknowledge that antigen detection with a rapid diagnostic test and molecular diagnosis by PCR may be useful in certain situations: “In the international setting, various test kits are available to detect antigens derived from malaria parasites. Such immunologic ("immunochromatographic") tests most often use a dipstick or cassette format and provide results in 2-15 minutes. These "Rapid Diagnostic Tests" (RDTs) offer a useful alternative to microscopy in situations where reliable microscopic diagnosis is not available. Malaria RDTs are currently used in some clinical settings and programs. On June 13, 2007, the U.S. Food and Drug Administration (FDA) approved the first RDT for use in the United States. This RDT is approved for use by clinical laboratories, not by individual clinicians or by patients themselves. It is recommended that all RDTs are followed-up with microscopy to confirm the results and if positive, to confirm the species and quantify the proportion of red blood cells that are infected. The use of this RDT may decrease the amount of time that it takes to determine whether a patient is infected with malaria. ... Parasite nucleic acids are detected using polymerase chain reaction (PCR). Although this technique may be more sensitive than blood smear microscopy, it is of limited utility for the diagnosis of acutely ill patients in the standard healthcare setting. PCR results are often not available quickly enough to be of value in establishing the diagnosis of malaria infection. PCR is most useful for confirming the species of malarial parasite after the diagnosis has been established by either smear microscopy or RDT” (CDC, 2024k).
Chikungunya (Staples et al., 2024): In the CDC Yellow Book, concerning the Chikungunya virus, they recommend that “the differential diagnosis of chikungunya virus infection depends on clinical features (signs and symptoms) as well as where the person was suspected of being infected. Consider other diseases in the differential diagnosis, including adenovirus, other alphaviruses (Barmah Forest, Mayaro, O’nyong-nyong, Ross River, and Sindbis), dengue, enterovirus, leptospirosis, malaria, measles, parvovirus, rubella, group A Streptococcus, typhus, Zika, and postinfectious arthritis and rheumatologic conditions. Laboratory diagnosis is done by serum testing for detection of virus, viral nucleic acid, or virus-specific IgM and neutralizing antibodies. Because the virus develops high levels of viremia during the first week after symptom onset, chikungunya can often be diagnosed by performing viral culture or nucleic acid amplification on serum. Virus-specific IgM antibodies normally develop toward the end of the first week of illness but can remain detectable for months to years after infection. Rarely, serum IgM antibody testing can yield false-positive results due to cross-reacting antibodies against related alphaviruses (e.g., Mayaro virus, O’nyong-nyong virus). …Testing for chikungunya virus is performed at several state health department laboratories, and commercial laboratories” (Staples et al., 2024).
West Nile Virus (WNV) (CDC, 2024o): “The front-line screening assay for laboratory diagnosis of human WNV infection is the IgM assay. Currently, the FDA has cleared three commercially available test kits from different manufacturers, for detection of WNV IgM antibodies…In addition, the CDC-defined IgM and IgG EIA [i.e., ELISA or microsphere-based immunoassay (MIA)] can be used. …The CDC MIA can differentiate WNV from St. Louis encephalitis. … Because the IgM and IgG antibody tests can cross-react between flaviviruses (e.g., [St. Louis encephalitis], dengue, yellow fever, WNV, Powassan), they should be viewed as screening tests only. For a case to be considered confirmed, serum samples that are antibody-positive on initial screening should be evaluated by a more specific test; currently the plaque reduction neutralization test (PRNT) is the recommended test for differentiating between flavivirus infections. … Specimens submitted for WNV testing should also be tested against other arboviruses known to be active or be present in the area or in the region where the patient traveled.”
There are also virus detection assays that can be utilized to detect viable WNV, WNV antigen or WNV RNA in human samples, but they vary in sensitivity, specificity, and time required to conduct the test. However, the CDC warns that “viremia is almost always absent by the time a patient presents with neuroinvasive illness and thus viral isolation is generally not recommended as part of a testing algorithm in immune competent patients. … Confirmation of virus isolate identity can be accomplished by indirect immunofluorescence assay (IFA) using virus-specific monoclonal antibodies (MAbs) or nucleic acid detection (e.g. RT-PCR, real-time RT-PCR or sequencing). … Virus isolation or RT-PCR on serum may be helpful in confirming WNV infection in immunocompromised patients when antibody development is delayed or absent” (CDC, 2024o).
Yellow Fever Virus (YFV) (Gershman & Staples, 2024): Isolation of the virus or NAAT should be performed as early as possible in suspected cases of YFV. “By the time more overt symptoms are recognized, the virus or viral RNA may no longer be detectable; thus, virus isolation and nucleic acid amplification should not be used to rule out a diagnosis of YF. Serologic assays can be used to detect virus-specific IgM and IgG antibodies. Because of the possibility of cross-reactivity between antibodies against other flaviviruses, however, more specific antibody testing (e.g., a plaque reduction neutralization test) should be performed to confirm the infection” (Gershman & Staples, 2024). Since YFV is a nationally notifiable disease, clinicians should contact their state and/or local health departments or call the CDC Arboviral Diseases Branch according to their respective local, state, and/or federal guidelines. As of May 2023, “Only one YF vaccine (YF-VAX, Sanofi Pasteur) is licensed for use in the United States. Periodically in the United States, shortages of YF-VAX have occurred due to production issues, including one that lasted from late 2015 until early 2021. To address this most recent shortage, Sanofi Pasteur collaborated with the CDC and the U.S. Food and Drug Administration (FDA) to import and distribute Stamaril (a YF vaccine comparable to YF-VAX, manufactured at the company’s facility in France) under an expanded-access investigational new drug protocol” (Gershman & Staples, 2024).
Dengue (CDC, 2024m): Diagnosis of dengue can be diagnosed differently based on the phase: the acute phase (0-7 days after symptom onset) and the convalescent phase (> 7 days after symptom onset). In the acute phase, the CDC recommends diagnosis using one of two testing combinations: “a nucleic acid amplification test (NAAT) (e.g., RT-PCR) and an IgM antibody test OR an NS1 antigen test and an IgM detection test,” but a serum sample is preferred in this stage. However, “a negative result from a RT-PCR or NS1 test does not rule out infection.” Furthermore, the CDC recommends that “when the acute (0 – 7 days) sample is negative in the recommended test combinations or is not available, a convalescent serum sample can be collected and tested.” For the convalescent sample, “IgM ELISA is recommended as the primary test after day 8 of symptom onset;” the CDC warns that after day 7 of illness, NAAT or NS1 antigen tests may not be as sensitive for disease detection.
The CDC does not recommend serologic testing by IgG for “diagnosis of acute dengue in patients, as these tests may detect antibodies from dengue infections or other flavivirus infections that occurred in the past.”
With regards to specific circumstances, “for people living in or traveling to an area with concurrently circulating flaviviruses, clinicians will need to order plaque reduction neutralization test (PRNT) to rule out dengue on IgM-positive specimens,” but PRNT does not always give a conclusive diagnostic result, “particularly in patients that have previously been exposed to more than one flavivirus.” Additionally, “if the patient is pregnant and symptomatic and lives in or has traveled to an area with risk of Zika, test for Zika using molecular tests in addition to dengue” (CDC, 2024m).
Zika Virus (CDC, 2024l): The CDC released updated guidelines associated with Zika testing for pregnant individuals. The recommendations for asymptomatic pregnant patients are shown below:
| Lived in or traveled to the United States and its territories during pregnancy |
Since no confirmed cases of Zika virus have been detected in the United States and its territories since 2018, routine Zika testing is not recommended. |
| Traveled to an area with an active CDC Zika Travel Health Notice during pregnancy |
NAAT testing may be considered up to 12 weeks after travel |
| Traveled to an area with current or past Zika virus transmission outside the U.S. and its territories during pregnancy |
Routine testing is not recommended. If the decision is made to test, NAAT testing can be done up to 12 weeks after travel. |
Recommendations for symptomatic pregnant patients are shown below:
| Lived in or traveled to an area with an active CDC Zika Travel Health Notice during pregnancy OR had sex during pregnancy with someone living in or with recent travel to an area with an active CDC Zika Travel Health Notice |
Specimens should be collected as soon as possible after onset of symptoms up to 12 weeks after symptom onset. Perform dengue and Zika virus NAAT and IgM testing on a serum specimen and Zika virus NAAT on a urine specimen. If Zika NAAT is positive and the Zika IgM is negative, repeat NAAT test on newly extracted RNA from same specimen to rule out false-positive results. If both dengue and Zika virus NAATs are negative but either IgM antibody test is positive, confirmatory PRNTs should be performed against dengue, Zika, and other flaviviruses endemic to the region where exposure occurred. |
| Lived in or traveled to an area with current or past Zika virus transmission during pregnancy |
Specimens should be collected as soon as possible after onset of symptoms up to 12 weeks after symptom onset. Perform dengue and Zika virus NAAT testing on a serum specimen and Zika virus NAAT on a urine specimen. If Zika NAAT is positive, repeat test on newly extracted RNA from same specimen to rule out false-positive results. Perform IgM testing for dengue only. If dengue NAAT or IgM test is positive, this provides adequate evidence of dengue infection, and no further testing is indicated. |
| Had sex during pregnancy with someone living in or with recent travel to an area with current or past Zika virus transmission |
Specimens should be collected as soon as possible after onset of symptoms up to 12 weeks after symptom onset. Only Zika NAAT should be performed. If Zika NAAT is positive, repeat test on newly extracted RNA from same specimen to rule out false-positive results. |
For pregnant patients having a fetus with prenatal ultrasound findings consistent with congenital Zika virus infection, the recommendations are below:
| Lived in or traveled during pregnancy to areas with an active CDC Zika Travel Health Notice or current or past Zika virus transmission OR had sex during pregnancy with someone living in or with recent travel to areas with an active CDC Zika Travel Health Notice or current or past Zika virus transmission |
Zika virus NAAT and IgM testing should be performed on pregnant person's serum and NAAT on pregnant person's urine. If the Zika virus NAATs are negative and the IgM is positive, confirmatory PRNTs should be performed against Zika and dengue. If amniocentesis is being performed as part of clinical care, Zika virus NAAT testing of amniocentesis specimens should also be performed and results interpreted within the context of the limitations of amniotic fluid testing. Testing of placental and fetal tissues may also be considered. |
For symptomatic non-pregnant patients, the recommendations are listed below:
| Living in or with recent travel to the United States and its territories |
Since no confirmed cases of Zika virus disease have been detected in the United States and its territories since 2018, routine Zika virus testing is not recommended. |
| Living in or with recent travel to an area with an active CDC Zika Travel Health Notice OR to an area with current or past Zika virus transmission outside the U.S. and its territories |
Dengue and Zika virus NAATs should be performed on serum collected ≤7 days after symptom onset. A positive NAAT result typically provides evidence of acute infection. Perform dengue and Zika virus IgM antibody testing on NAAT-negative serum specimens and serum collected >7 days after onset of symptoms. If either dengue or Zika virus IgM antibody testing is positive, and definitive diagnosis is needed for clinical or epidemiologic purposes, confirmatory PRNTs should be performed against dengue, Zika, and other flaviviruses endemic to the region where exposure occurred. |
For infants with possible congenital Zika virus infection via gestational parents with possible Zika virus exposure during pregnancy, the CDC recommends to:
- “Collect specimens as soon as possible after birth.
- Zika virus NAAT and IgM testing should be performed on infant serum and NAAT on infant urine.
- If cerebrospinal fluid (CSF) is obtained for other purposes, NAAT and IgM antibody testing should be performed on CSF.
- If the infant’s serum is IgM non-negative and NAAT negative, but PRNT was not performed on the gestational parent’s serum, PRNT for Zika and dengue viruses should be performed on the infant serum.
- Perform PRNT on a sample collected from an infant aged 18 months or older whose initial sample collected at birth was IgM non-negative and neutralizing antibodies were detected by PRNT in either the infant’s or gestational parent’s sample.”
For asymptomatic non-pregnant patients, “testing for dengue or Zika viruses is not recommended for this group” (CDC, 2024l).
Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (American Society of Microbiology)
Laboratory Diagnosis of Tickborne Infections: The information given below outlines the diagnostic procedures for tickborne infections and is taken from Table 50 of the 2024 IDSA/ASM guidelines.
| Etiologic Agents |
Diagnostic Procedures |
Optimum Specimens |
| Bacteria |
||
| Relapsing fever borreliae Borrelia hermsii (western USA) Borrelia parkeri (western USA) Borrelia turicatae (southwestern USA) Borrelia mazzottii (southern USA) |
Primary test: Wright’s, Giemsa, or Diff-Quik stains of peripheral thin or/ and thick blood smears. Can be seen in direct wet preparation of blood in some cases. |
Blood or bone marrow |
| Other testing: NAAT, Serologic testing |
Serum, blood or body fluids for NAAT. Serum for culture or serologic testing. |
|
| Borrelia burgdorferi sensu lato complex (Lyme borreliosis) Borrelia burgdorferi (USA) Borrelia mayonii (USA) Borrelia garinii (Europe, Asia) Borrelia afzelii (Europe, Asia) |
Early, localized Lyme disease with erythema migrans (EM) Testing not routinely recommended |
Not applicable |
| Early if disseminated: If EM or multiple EM rash absent (weeks through months after tick bite) or late (months through years after tick bite) in untreated patients: Primary test: Two-tier testing (acute- and convalescent-phase sera optimal) = EIA antibody screening. If EIA result is positive or equivocal, supplemental IgM/IgG immunoblots or EIAs are required NOTE: Immunoblot or supplemental EIAs should NOT be performed unless an initial EIA is reported as positive or equivocal. |
Serum |
|
| Early Lyme Neuroborreliosis: Two-tiered testing algorithm Late Lyme Neuroborreliosis CSF/Serum Antibody Index |
Serum
Paired serum and CSF, collected within 24 hours |
|
| NAAT |
Biopsy specimens of infected skin, synovial fluid or tissue, etc. |
|
| Borrelia miyamotoi (B. miyamotoi infection, hard tick-borne relapsing fever) |
Primary test for acute infection: NAAT |
Blood |
| Serology: EIA for detection of antibodies to recombinant GlpQ antigen |
Serum |
|
| Anaplasma phagocytophilum (human granulocytotropic anaplasmosis) |
Primary test for acute infection: NAAT Alternate Primary (if experienced technologists available/NAAT is unavailable): Wright or Giemsa stain of peripheral blood or buffy coat leukocytes during week first week of infection. |
Blood
|
| Serology: Acute and convalescent IFA titers for IgG-class antibodies to A. phagocytophilum antibodies |
Serum |
|
| Immunohistochemical staining of Anaplasma antigens in formalin-fixed, paraffin-embedded specimens |
Bone marrow biopsies or autopsy tissues (spleen, lymph nodes, liver, and lung) |
|
| Ehrlichia chaffeensis (human monocytotropic ehrlichiosis) Ehrlichia muris Ehrlichia ewingii |
Primary test for acute infection: NAAT NOTE: Only definitive diagnostic assay for E. ewingii Wright or Giemsa stain of peripheral blood or buffy coat leukocytes smear during first week of infection |
Whole blood for NAAT
Blood for Wright or Giemsa stain |
| Serology: acute and convalescent IFA titers for Ehrlichia IgG-class antibodies NOTE: Not recommended for acute infection |
Serum |
|
| Immunohistochemical staining of Ehrlichia antigens in formalin-fixed, paraffin-embedded specimens |
Bone marrow biopsies or autopsy tissues (spleen, lymph nodes, liver and lung) |
|
| Rickettsia rickettsii (RMSF) Other spotted fever group Rickettsia spp (mild spotted fever) R. typhi (murine typhus) R. akari (rickettsialpox) R. prowazekii (epidemic typhus) |
Serology: acute and convalescent IFA for Rickettsia sp. IgM and IgG antibodies |
Serum
|
| NAAT |
Skin biopsy (preferably a maculopapule containing petechiae or the margin of an eschar) or autopsy tissues (liver, spleen, lung, heart, and brain) |
|
| Immunohistochemical staining of spotted fever group rickettsiae antigens (up to first 24 h after antibiotic therapy initiated) in formalin-fixed, paraffin-embedded specimens |
Skin biopsy (preferably a maculopapule containing petechiae or the margin of an eschar) or autopsy tissues (liver, spleen, lung, heart, and brain) |
|
| Protozoa |
||
| Babesia microti Babesia sp. |
Primary test: Giemsa, Wright’s, Wright-Giemsa stains of peripheral thin and thick blood smears (Giemsa preferred) |
Whole blood (EDTA vacutainer tube is a second choice) |
| Primary test for acute infection: NAAT |
Blood |
|
| Serology: acute and convalescent IFA titers for Babesia IgG-class antibodies NOTE: Not recommended for acute infection. |
Serum
|
|
| Virus |
||
| Dengue Virus |
Serology NS1 Antigen |
Serum |
| NAAT |
CSF, plasma, serum |
|
| West Nile Virus and Other Endemic Arboviruses in North America |
Serology |
Serum |
| NAAT |
CSF, plasma, serum |
|
| Zika Virus |
Serology |
CSF, serum |
| NAAT |
CSF, plasma, serum, urine, whole blood |
|
The IDSA/ASM does note that most PCR-based assays for babesiosis only detect B. microti even though there are at least three other species of Babesia that can cause the infection. “Real time PCR available from CDC and reference labs… Serology does not distinguish between acute and past infection” (Miller et al., 2024).
Their recommendation for the main diagnostic testing for malaria due to Plasmodium falciparum, P. ovale, P. vivax, P. malariae, and P. knowlesi is “STAT microscopic examination of Giemsa-stained thick and thin blood films (repeat testing every 12 – 24 h for a total of 3 exams before ruling out malaria); rapid antigen detection tests followed by confirmatory blood films within 12 – 24 h.” They make the following special remark: “Antigen tests lack sensitivity with low parasitemia and non-falciparum malaria and do not differentiate all species. PCR from some reference laboratories will detect and differentiate all species. Calculation of percent parasitemia and species identification (using thick or thin blood films) is required for determining patient management and following response to therapy” (Miller et al., 2024). Concerning DENV, “Plaque reduction neutralization tests (PRNTs) are considered the reference standard for detection of antibodies to arthropod-borne viruses (arboviruses) and provide improved specificity over commercial serologic assays; however, due to the complexity of testing, PRNT is currently only available at select public health laboratories and the CDC.” They note that false positives for antibodies to DENV may not necessarily indicate DENV infection since it can also be indicative of a prior flavivirus infection, such as West Nile virus, SLE, or Zika virus. They also state that the “Detection of DENV RNA by NAAT is preferred for acutely ill patients presenting within 7 days of symptom onset. Recently, detection of the DENV NS1 antigen, which is secreted from infected host cells as early as 1 day after symptom onset and up to 10 days thereafter, has become an acceptable alternative to NAAT for diagnosis of acute DENV infection” (Miller et al., 2024).
For West Nile Virus (WNV), they state: “Laboratory diagnosis of these arboviruses is typically accomplished by detecting virus-specific IgM- and/or IgG-class antibodies in serum and/or CSF.” Additionally, “However, introduction of blood into the CSF during a traumatic lumbar puncture or defective permeability of the blood-brain barrier may lead to falsely elevated IgM levels in the CSF. Importantly, antibody cross-reactivity among the flaviviruses is not uncommon when using ELISA or IFA-based assays” (Miller et al., 2024).
World Health Organization (WHO)
Interim guidance for laboratory testing of Zika and dengue virus published in July 2022 by WHO includes these updated key considerations, recommendations, and good practices:
- ZIKV and DENV infections need to be differentiated from each other, and from other circulating arboviral and non-arboviral pathogens, using laboratory tests.
- Laboratory tests performed and interpretation of results must be guided by the interval between symptom onset or exposure, and the collection of specimens.
- WHO recommends the use of whole blood, serum, or plasma routine diagnostic testing for arboviruses, and urine for ZIKV NAAT testing.
- Molecular assays are the preferred detection method but the period of RNA detectability following infection is limited.
- Interpretation of serologic test results remains challenging because of cross-reactivity and prolonged detection of virus-specific antibodies; their utility depends on the patient’s current and prior flavivirus exposures.
- Testing for antibodies to ZIKV and DENV should thus be done with careful consideration of epidemiologic and clinical context.
- For pregnant women, the diagnosis of ZIKV should always be based on laboratory evidence and testing in these patients should not be limited to a subset of samples, even during outbreaks.
- For pregnant women, accurate diagnosis is of particular importance; prolonged detection of RNA in blood and urine may facilitate. confirmation of ZIKV infection in these patients
- ZIKV IgM testing in pregnant women should be used with caution, since a positive test might reflect infection that occurred prior to pregnancy
- ZIKV testing for asymptomatic pregnant women remains challenging because of unknown optimal timing of specimen collection and risks of false positive and false negative results.
- Only laboratory tests that have undergone independent, comprehensive assessment of quality, safety and performance should be used for diagnosing arboviral infections.
- Any testing for the presence of ZIKV, DENV, and other pathogens in the differential diagnosis should be performed in appropriately equipped laboratories by staff trained in the relevant technical and safety procedures (WHO, 2022a)
American Society for Microbiology (American Society of Microbiology)
The ASM updated guidelines in 2022 on laboratory testing for Zika virus. They state, “Diagnostic testing may be warranted for patients who live in or have recently travelled to an endemic region and are critically ill, hospitalized or pregnant, or infants born to Zika virus positive mothers” (American Society of Microbiology, 2022). The ASM endorses CDC guidelines on Zika as well.
American Academy of Pediatrics 2021-2024 Redbook
Babesiosis (American Academy of Pediatrics, 2021a): “Acute, symptomatic cases of babesiosis typically are diagnosed by microscopic identification of Babesia parasites on Giemsa- or Wright-stained blood smears. … If the diagnosis of babesiosis is being considered, manual (nonautomated) review of blood smears for parasites should be requested explicitly. If seen, the tetrad (Maltese-cross) form is pathognomonic. B microti and other Babesia species can be difficult to distinguish … examination of blood smears by a reference laboratory should be considered for confirmation of the diagnosis.” They do state that antibody testing can be useful in distinguishing between Babesia and Plasmodium infections whenever blood smear examinations and travel histories are inconclusive or for detecting individuals with very low levels of parasitemia.
Non-Lyme Borrelia Infections (American Academy of Pediatrics, 2021b): Dark-field microscopy and Wright-, Giemsa-, or acridine orange-stained preparations of blood smears can be used to observe the presence of spirochetes in the initial febrile episode, but their presence is more difficult to determine in future recurrences. Both enzyme immunoassay and Western immunoblot analysis can detect serum antibodies; however, “Antibody tests are not standardized and are affected by antigenic variations among and within Borrelia species and strains.” As of publication, PCR and antibody-based testing were still under development and were not widely available.
Ehrlichia, Anaplasma, and Related Infections (American Academy of Pediatrics, 2021e): PCR testing should be performed within the first week of illness to diagnose anaplasmosis, ehrlichiosis, and other Anaplasmataceae infections because doxycycline treatment rapidly decreases the sensitivity of PCR. Consequently, negative PCR results do not necessarily indicate a lack of infection. Occasionally, Giemsa- or Wright staining of blood smears can be performed to identify the presence of the morulae of Anaplasma in the first week of illness. Culture testing for isolation is not performed. “Serologic testing may be used to demonstrate a fourfold change in immunoglobulin (Ig) G-specific antibody titer by indirect immunofluorescence antibody (IFA) assay between paired acute and convalescent specimens taken 2 to 4 weeks apart. A single mildly elevated IgG titer may not be diagnostic, particularly in regions with high prevalence. IgM serologic assays are prone to false-positive reactions, and IgM can remain elevated for lengthy periods of time, reducing its diagnostic utility.”
Rocky Mountain Spotted Fever (RMSF) (American Academy of Pediatrics, 2021h): “The gold standard confirmatory test is indirect immunofluorescence antibody (IFA) to R rickettsii antigen. Both immunoglobulin (Ig) G and IgM antibodies begin to increase around 7 to 10 days after onset of symptoms; IgM is less specific, and IgG is the preferred test. Confirmation requires a fourfold or greater increase in antigen-specific IgG between acute (first 1 – 2 weeks of illness while symptomatic) and convalescent (2 – 4 weeks later) sera.”
Rickettsialpox (American Academy of Pediatrics, 2021g): Rickettsialpox can be mistaken for other rickettsial infections. Ideally, the use of R. akari-specific antigen is recommended for serologic diagnosis, but it has limited availability. Otherwise, indirect IFA for R. rickettsia, the causative agent of RMSF, since R. akari has extensive cross-reactivity. Again, a demonstration of at least a fourfold increase in antibody titers taken two to six weeks apart is indicative of infection.
Chikungunya (American Academy of Pediatrics, 2021c): “Laboratory diagnosis generally is accompanied by testing serum to detect virus, viral nucleic acid, or virus-specific immunoglobulin (Ig) M and neutralizing antibodies.” RT-PCR can be used to diagnose chikungunya during the first week after onset of symptoms since chikungunya-specific antibodies have not formed at that time. After the first week, serum testing of IgM or a plaque reduction neutralization test can be performed.
Dengue (American Academy of Pediatrics, 2021d): “Dengue virus is detectable by RT-PCR or NS1 antigen EIAs from the beginning of the febrile phase until day 7 to 10 after illness onset.” Cross-reactivity occurs between anti-dengue virus IgM and other flaviviruses, including Zika. IgG EIA and hemagglutination testing is not specific for diagnosis of dengue, and IgG antibodies remain elevated for life; consequently, a fourfold increase in IgG between the acute and convalescent phase can confirm recent infection, with “Reference testing is available from the Dengue Branch of the Centers for Disease Control and Prevention.”
Malaria (American Academy of Pediatrics, 2021f): Microscopic identification of Plasmodium on both thick and thin blood films should be performed. “If initial blood smears test negative for Plasmodium species but malaria remains a possibility, the smear should be repeated every 12 to 24 hours during a 72-hour period. … Serologic testing generally is not helpful, except in epidemiologic surveys… Species confirmation and antimalarial drug resistance testing are available free of charge at the Centers for Disease Control and Prevention (CDC) for all cases of malaria diagnosed in the United States.” One FDA approved RADT is available in the U.S. to hospitals and commercial labs; however, both positive and negative test results must be corroborated by microscopic examination.
West Nile Virus (WNV) (American Academy of Pediatrics, 2021i): PCR is not recommended for diagnosis of WNV in immunocompetent patients since WNV RNA is usually no longer detectable by the initial onset of symptoms. “Detection of anti-WNV immunoglobulin (Ig) M antibodies in serum or CSF is the most common way to diagnose WNV infection.” Anti-WNV IgM levels can remain elevated for longer than one year so a positive test result may be indicative of a prior infection. “Plaque-reduction neutralization tests can be performed to measure virus-specific neutralizing antibodies and to discriminate between cross-reacting antibodies from closely related flaviviruses. A fourfold or greater increase in virus-specific neutralizing antibodies between acute-and convalescent-phase serum specimens collected 2 or 3 weeks apart may be used to confirm recent WNV infection.”
International Encephalitis Consortium (IEC)
In 2013, the IEC released their Case Definitions, Diagnostic Algorithms, and Priorities in Encephalitis. Concerning arboviruses, they state the following: “For most arboviruses, serologic testing of serum and CSF is preferred to molecular testing, since the peak of viremia typically occurs prior to symptom onset. For example, in patients with West Nile virus (WNV) associated with neuroinvasive disease, CSF PCR is relatively insensitive (57%) compared with detection of WNV IgM in CSF. The cumulative percentage of seropositive patients increases by approximately 10% per day during the first week of illness suggesting the need for repeat testing if the suspicion for disease is strong in those with initially negative results. Notably, arbovirus IgM antibodies may be persistently detectable in the serum and, less commonly, in the CSF, for many months after acute infection, and therefore may not be indicative of a current infection. Therefore, if possible, documentation of acute infection by seroconversion and/or 4-fold or greater rises in titre using paired sera is recommended” (Venkatesan et al., 2013).
References
- Akoolo, L., Schlachter, S., Khan, R., Alter, L., Rojtman, A. D., Gedroic, K., Bhanot, P., & Parveen, N. (2017). A novel quantitative PCR detects Babesia infection in patients not identified by currently available non-nucleic acid amplification tests. BMC Microbiol, 17(1), 16. https://doi.org/10.1186/s12866-017-0929-2
- American Academy of Pediatrics. (2021a). Babesiosis. In D. Kimberlin, M. Brady, M. Jackson, & S. Long (Eds.), Red Book: 2021- 2024 Report of the Committee on Infectious Diseases (pp. 235-237). American Academy of Pediatrics. https://redbook.solutions.aap.org/chapter.aspx?sectionid=189640045&bookid=2205
- American Academy of Pediatrics. (2021b). Borrelia Infections Other Than Lyme Disease. In D. Kimberlin, M. Brady, M. Jackson, & S. Long (Eds.), Red Book: 2021- 2021 Report of the Committee on Infectious Diseases (pp. 252-255). American Academy of Pediatrics. https://redbook.solutions.aap.org/chapter.aspx?sectionid=189640055&bookid=2205
- American Academy of Pediatrics. (2021c). Chikungunya. In D. Kimberlin, M. Brady, M. Jackson, & S. Long (Eds.), Red Book: 2021-2024 Report of the Committee on Infectious Diseases (pp. 271-272). American Academy of Pediatrics. https://redbook.solutions.aap.org/chapter.aspx?sectionId=189640062&bookId=2205
- American Academy of Pediatrics. (2021d). Dengue. In D. Kimberlin, M. Brady, M. Jackson, & S. Long (Eds.), Red Book: 2021-2024 Report of the Committee on Infectious Diseases (pp. 317-319). American Academy of Pediatrics. https://redbook.solutions.aap.org/chapter.aspx?sectionId=189640081&bookId=2205
- American Academy of Pediatrics. (2021e). Ehrlichia, Anaplasma, and Related Infections. In D. Kimberlin, M. Brady, M. Jackson, & S. Long (Eds.), Red Book: 2021-2024 Report of the Committee on Infectious Diseases (pp. 323-328). American Academy of Pediatrics. https://redbook.solutions.aap.org/chapter.aspx?sectionid=189640084&bookid=2205
- American Academy of Pediatrics. (2021f). Malaria. In D. Kimberlin, M. Brady, M. Jackson, & S. Long (Eds.), Red Book: 2021-2024 Report of the Committee on Infectious Diseases (pp. 527-537). American Academy of Pediatrics. https://redbook.solutions.aap.org/chapter.aspx?sectionId=189640129&bookId=2205
- American Academy of Pediatrics. (2021g). Rickettsialpox. In D. Kimberlin, M. Brady, M. Jackson, & S. Long (Eds.), Red Book: 2021-2024 Report of the Committee on Infectious Diseases (pp. 696-697). American Academy of Pediatrics. https://redbook.solutions.aap.org/chapter.aspx?sectionId=189640173&bookId=2205
- American Academy of Pediatrics. (2021h). Rocky Mountain Spotted Fever. In D. Kimberlin, M. Brady, M. Jackson, & S. Long (Eds.), Red Book: 2021-2024 Report of the Committee on Infectious Diseases (pp. 697-700). American Academy of Pediatrics. https://redbook.solutions.aap.org/chapter.aspx?sectionid=189640174&bookid=2205
- American Academy of Pediatrics. (2021i). West Nile Virus. In D. Kimberlin, M. Brady, M. Jackson, & S. Long (Eds.), Red Book: 2021 - 2024 Report of the Committee on Infectious Diseases (pp. 888-891). American Academy of Pediatrics. https://redbook.solutions.aap.org/chapter.aspx?sectionId=189640220&bookId=2205
- American Society of Microbiology. (2022, September 20, 2022). Zika Virus: An Update on the Disease and Guidance for Laboratory Testing. Retrieved March 31 from https://asm.org/Guideline/Zika-virus-An-update-on-the-disease-and-guidance-f
- Barbour, A. G. (2024, May 29). Clinical features, diagnosis, and management of relapsing fever. https://www.uptodate.com/contents/clinical-features-diagnosis-and-management-of-relapsing-fever
- Biggs, H. M., Behravesh, C. B., Bradley, K. K., Dahlgren, F. S., Drexler, N. A., Dumler, J. S., Folk, S. M., Kato, C. Y., Lash, R. R., Levin, M. L., Massung, R. F., Nadelman, R. B., Nicholson, W. L., Paddock, C. D., Pritt, B. S., & Traeger, M. S. (2016). Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis - United States. MMWR Recomm Rep, 65(2), 1-44. https://doi.org/10.15585/mmwr.rr6502a1
- Burakoff, A., Lehman, J., Fischer, M., Staples, J. E., & Lindsey, N. P. (2018). West Nile Virus and Other Nationally Notifiable Arboviral Diseases - United States, 2016. MMWR Morb Mortal Wkly Rep, 67(1), 13-17. https://doi.org/10.15585/mmwr.mm6701a3
- Calisher, C. H. (1994). Medically important arboviruses of the United States and Canada. Clin Microbiol Rev, 7(1), 89-116.
- CDC. (2024a, May 15). About Hard Tick Relapsing Fever (HTRF). https://www.cdc.gov/relapsing-fever/about/about-htrf.html
- CDC. (2024b, May 15). About Louse-Borne Relapsing Fever (LBRF). https://www.cdc.gov/relapsing-fever/about/about-lbrf.html
- CDC. (2024c, May 15). About Soft Tick Relapsing Fever (STRF). https://www.cdc.gov/relapsing-fever/about/about-strf.html
- CDC. (2024d, 05/15/2024). Clinical and Laboratory Diagnosis for Rocky Mountain Spotted Fever. https://www.cdc.gov/rocky-mountain-spotted-fever/hcp/diagnosis-testing/
- CDC. (2024e, 05/15/2024). Clinical Features and Diagnosis of Colorado Tick Fever. https://www.cdc.gov/colorado-tick-fever/hcp/clinical-diagnosis/
- CDC. (2024f, 06/05/2024). Clinical Features of Dengue. https://www.cdc.gov/dengue/hcp/clinical-signs/
- CDC. (2024g, May 14 ). Clinical Guidance for Hard Tick Relapsing Fever (HTRF). https://www.cdc.gov/relapsing-fever/hcp/hard-tick-relapsing-fever/index.html
- CDC. (2024h, May 14). Clinical Guidance for Louse-borne Relapsing Fever (LBRF). https://www.cdc.gov/relapsing-fever/hcp/louse-borne-relapsing-fever/index.html
- CDC. (2024i, May 14). Clinical Guidance for Soft Tick Relapsing Fever (STRF). https://www.cdc.gov/relapsing-fever/hcp/soft-tick-relapsing-fever/
- CDC. (2024j, February 13). Clinical Overview of Babesiosis. https://www.cdc.gov/babesiosis/hcp/clinical-overview/
- CDC. (2024k, 03/20/2024). Clinical Testing and Diagnosis for Malaria. https://www.cdc.gov/malaria/hcp/diagnosis-testing/index.html
- CDC. (2024l, May 14). Clinical Testing and Diagnosis for Zika Virus Disease. https://www.cdc.gov/zika/hcp/diagnosis-testing/
- CDC. (2024m, May 31). Clinical Testing Guidance for Dengue. https://www.cdc.gov/dengue/hcp/diagnosis-testing/
- CDC. (2024n, 05/31/2024). Congenital Zika Syndrome and Other Birth Defects. https://www.cdc.gov/zika/czs/
- CDC. (2024o, May 31). Guidelines for West Nile Virus Surveillance and Control. https://www.cdc.gov/west-nile-virus/php/surveillance-and-control-guidelines/index.html
- CDC. (2024p). Relapsing Fever. Centers for Disease Control and Prevention. https://www.cdc.gov/relapsing-fever/hcp/soft-tick-relapsing-fever/?CDC_AAref_Val=https://www.cdc.gov/relapsing-fever/clinicians/index.html
- CDC. (2024q, May 15). Rocky Mountain Spotted Fever. Centers for Disease Control and Prevention. https://www.cdc.gov/rocky-mountain-spotted-fever/data-research/facts-stats/index.html
- CDC. (2024r, 05/14/2024). Symptoms of Dengue and Testing. https://www.cdc.gov/dengue/signs-symptoms/index.html
- CDC. (2024s, 05/08/2024). A—Z Index of Vector-Borne Diseases and Conditions. https://www.cdc.gov/vector-borne-diseases/about/a-z-index-of-vector-borne-diseases.html
- CDC. (2024t, 05/31/2024). Zika Symptoms and Complications. https://www.cdc.gov/zika/signs-symptoms/
- Clinic, M. (2024). Test ID: LCMAL Malaria, Molecular Detection, PCR, Varies. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/87860
- Cohee, L., & Seydel, K. (2022, October 17). Malaria: Clinical manifestations and diagnosis in nonpregnant adults and children. https://www.uptodate.com/contents/malaria-clinical-manifestations-and-diagnosis-in-nonpregnant-adults-and-children
- Denison, A. M., Amin, B. D., Nicholson, W. L., & Paddock, C. D. (2014). Detection of Rickettsia rickettsii, Rickettsia parkeri, and Rickettsia akari in skin biopsy specimens using a multiplex real-time polymerase chain reaction assay. Clin Infect Dis, 59(5), 635-642. https://doi.org/10.1093/cid/ciu358
- Dimaio, M. A., Pereira, I. T., George, T. I., & Banaei, N. (2012). Performance of BinaxNOW for diagnosis of malaria in a U.S. hospital. J Clin Microbiol, 50(9), 2877-2880. https://doi.org/10.1128/jcm.01013-12
- FDA. (2018). Devices@FDA. U.S. Department of Health & Human Services. Retrieved 08/06/2018 from https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm
- Gershman, M., & Staples, J. (2024). CDC Yellow Book 2024 Travel-Associated Infections & Diseases: Yellow Fever. In G. Brunette (Ed.), CDC Yellow Book 2024: Travel-Associated Infections & Diseases. Oxford University Press. https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/yellow-fever
- Granger, D., & Theel, E. S. (2019). Evaluation of a Rapid Immunochromatographic Assay and Two Enzyme-Linked Immunosorbent Assays for Detection of IgM-Class Antibodies to Zika Virus. J Clin Microbiol, 57(3). https://doi.org/10.1128/jcm.01413-18
- Hopkins, H. (2023, Feb. 2023). Laboratory tools for the diagnosis of malaria. Wolters Kluwer. https://www.uptodate.com/contents/diagnosis-of-malaria
- Johnson, A. J., Martin, D. A., Karabatsos, N., & Roehrig, J. T. (2000). Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J Clin Microbiol, 38(5), 1827-1831.
- Kalish, R. A., McHugh, G., Granquist, J., Shea, B., Ruthazer, R., & Steere, A. C. (2001). Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10-20 years after active Lyme disease. Clin Infect Dis, 33(6), 780-785. https://doi.org/10.1086/322669
- Kato, C. Y., Chung, I. H., Robinson, L. K., Austin, A. L., Dasch, G. A., & Massung, R. F. (2013). Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol, 51(1), 314-317. https://doi.org/10.1128/jcm.01723-12
- Kim, Y. H., Lee, J., Kim, Y.-E., Chong, C.-K., Pinchemel, Y., Reisdörfer, F., Coelho, J. B., Dias, R. F., Bae, P. K., Gusmão, Z. P. M., Ahn, H.-J., & Nam, H.-W. (2018). Development of a Rapid Diagnostic Test Kit to Detect IgG/IgM Antibody against Zika Virus Using Monoclonal Antibodies to the Envelope and Non-structural Protein 1 of the Virus. The Korean journal of parasitology, 56(1), 61-70. https://doi.org/10.3347/kjp.2018.56.1.61
- Krause, P. J., & Vannier, E. G. (2024, Aug 2). Babesiosis: Clinical manifestations and diagnosis. https://www.uptodate.com/contents/babesiosis-clinical-manifestations-and-diagnosis
- LeBeaud, A. D. (2023, December 8). Zika virus infection: An overview. https://www.uptodate.com/contents/zika-virus-infection-an-overview
- Leski, T. A., Taitt, C. R., Swaray, A. G., Bangura, U., Reynolds, N. D., Holtz, A., Yasuda, C., Lahai, J., Lamin, J. M., Baio, V., Jacobsen, K. H., Ansumana, R., & Stenger, D. A. (2020). Use of real-time multiplex PCR, malaria rapid diagnostic test and microscopy to investigate the prevalence of Plasmodium species among febrile hospital patients in Sierra Leone. Malaria Journal, 19(1), 84. https://doi.org/10.1186/s12936-020-03163-2
- Mathison, B. A., & Pritt, B. S. (2017). Update on Malaria Diagnostics and Test Utilization. J Clin Microbiol, 55(7), 2009-2017. https://doi.org/10.1128/jcm.02562-16
- McClain, M. T. (2024a, February 16). Epidemiology, clinical manifestations, and diagnosis of Rocky Mountain spotted fever. https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-rocky-mountain-spotted-fever
- McClain, M. T. (2024b, April 19). Human ehrlichiosis and anaplasmosis. https://www.uptodate.com/contents/human-ehrlichiosis-and-anaplasmosis
- McClain, M. T. (2024c, January 31). Other spotted fever group rickettsial infections. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- McQuiston, J. H., Wiedeman, C., Singleton, J., Carpenter, L. R., McElroy, K., Mosites, E., Chung, I., Kato, C., Morris, K., Moncayo, A. C., Porter, S., & Dunn, J. (2014). Inadequacy of IgM antibody tests for diagnosis of Rocky Mountain Spotted Fever. The American journal of tropical medicine and hygiene, 91(4), 767-770. https://doi.org/10.4269/ajtmh.14-0123
- Meatherall, B., Preston, K., & Pillai, D. R. (2014). False positive malaria rapid diagnostic test in returning traveler with typhoid fever. BMC Infect Dis, 14, 377. https://doi.org/10.1186/1471-2334-14-377
- Miller, J. M., Binnicker, M. J., Campbell, S., Carroll, K. C., Chapin, K. C., Gonzalez, M. D., Harrington, A., Jerris, R. C., Kehl, S. C., Leal, S. M., Jr., Patel, R., Pritt, B. S., Richter, S. S., Robinson-Dunn, B., Snyder, J. W., Telford, S., 3rd, Theel, E. S., Thomson, R. B., Jr., Weinstein, M. P., & Yao, J. D. (2024). Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2024 Update by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. https://doi.org/10.1093/cid/ciae104
- Nasci, R., Fischer, M., Lindsey, N., Lanciotti, R., Savage, H., Komar, N., McAllister, J., Mutebi, J.-P., Lavelle, J., Zielinski-Gutierrez, E., & Petersen, L. (2013). West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. https://www.cdc.gov/west-nile-virus/php/surveillance-and-control-guidelines/index.html
- Ota-Sullivan, K., & Blecker-Shelly, D. L. (2013). Use of the rapid BinaxNOW malaria test in a 24-hour laboratory associated with accurate detection and decreased malaria testing turnaround times in a pediatric setting where malaria is not endemic. J Clin Microbiol, 51(5), 1567-1569. https://doi.org/10.1128/jcm.00293-13
- Petersen, L. R. (2021, September 13). Arthropod-borne encephalitides. https://www.uptodate.com/contents/arthropod-borne-encephalitides
- Petersen, L. R. (2022, August 4). Clinical manifestations and diagnosis of West Nile virus infection. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-west-nile-virus-infection
- Reynolds, M. R., Jones, A. M., Petersen, E. E., Lee, E. H., Rice, M. E., Bingham, A., Ellington, S. R., Evert, N., Reagan-Steiner, S., Oduyebo, T., Brown, C. M., Martin, S., Ahmad, N., Bhatnagar, J., Macdonald, J., Gould, C., Fine, A. D., Polen, K. D., Lake-Burger, H., . . . Honein, M. A. (2017). Vital Signs: Update on Zika Virus-Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure - U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep, 66(13), 366-373. https://doi.org/10.15585/mmwr.mm6613e1
- Rosenberg, R., Lindsey, N. P., Fischer, M., Gregory, C. J., Hinckley, A. F., Mead, P. S., Paz-Bailey, G., Waterman, S. H., Drexler, N. A., Kersh, G. J., Hooks, H., Partridge, S. K., Visser, S. N., Beard, C. B., & Petersen, L. R. (2018). Vital Signs: Trends in Reported Vectorborne Disease Cases - United States and Territories, 2004-2016. MMWR Morb Mortal Wkly Rep, 67(17), 496-501. https://doi.org/10.15585/mmwr.mm6717e1
- Shiu, C., Starker, R., Kwal, J., Bartlett, M., Crane, A., Greissman, S., Gunaratne, N., Lardy, M., Picon, M., Rodriguez, P., Gonzalez, I., & Curry, C. L. (2018). Zika Virus Testing and Outcomes during Pregnancy, Florida, USA, 2016. Emerg Infect Dis, 24(1), 1-8. https://doi.org/10.3201/eid2401.170979
- Staples, J., Hills, S., & Powers, A. (2024). CDC Yellow Book 2024 Travel-Associated Infections & Diseases: Chikungunya. In G. Brunette (Ed.), CDC Yellow Book 2024: Travel-Associated Infections & Diseases. Oxford University Press. https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/chikungunya
- Tan, K., & Abanyie, F. (2024). CDC Yellow Book 2024 Travel-Associated Infections & Diseases: Malaria. In CDC Yellow Book: Health Information for International Travel. Oxford University Press. https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/malaria
- Thomas, S., Rothman, A., Srikiatkhachorn, A., & Kalayanarooj, S. (2022, October 5). Dengue virus infection: Clinical manifestations and diagnosis. https://www.uptodate.com/contents/dengue-virus-infection-clinical-manifestations-and-diagnosis
- van Bergen, K., Stuitje, T., Akkers, R., Vermeer, E., Castel, R., & Mank, T. (2021). Evaluation of a novel real-time PCR assay for the detection, identification and quantification of Plasmodium species causing malaria in humans. Malar J, 20(1), 314. https://doi.org/10.1186/s12936-021-03842-8
- Venkatesan, A., Tunkel, A. R., Bloch, K. C., Lauring, A. S., Sejvar, J., Bitnun, A., Stahl, J. P., Mailles, A., Drebot, M., Rupprecht, C. E., Yoder, J., Cope, J. R., Wilson, M. R., Whitley, R. J., Sullivan, J., Granerod, J., Jones, C., Eastwood, K., Ward, K. N., . . . International Encephalitis, C. (2013). Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis, 57(8), 1114-1128. https://doi.org/10.1093/cid/cit458
- WHO. (2022a). Laboratory testing for Zika virus and dengue virus infections https://www.who.int/publications/i/item/WHO-ZIKV_DENV-LAB-2022.1
- WHO. (2022b, 10/31/2017). Plague. World Health Organization. Retrieved 08/10/2018 from http://www.who.int/news-room/fact-sheets/detail/plague
- Wilder-Smith, A. (2024, June 11). Yellow fever: Epidemiology, clinical manifestations, and diagnosis. https://www.uptodate.com/contents/yellow-fever-epidemiology-clinical-manifestations-and-diagnosis
- Wilson, M. E., & Lenschow, D. J. (2022, January 24). Chikungunya fever: Epidemiology, clinical manifestations, and diagnosis. https://www.uptodate.com/contents/chikungunya-fever-epidemiology-clinical-manifestations-and-diagnosis
Coding Section
| Code |
Number |
Description |
| CPT |
86757 |
Antibody; Rickettsia; Immunoassay Indirect Fluorescent Antibody (IFA) |
| 87040 | Culture, bacterial; blood, aerobic, with isolation and presumptive identification of Infectious agent detection by nucleic acid (DNA or RNA), not otherwise specified; | |
| 86280 | Hemagglutination inhibition test (HAI) | |
|
|
86382 |
Neutralization test, viral |
|
|
86753 |
IFA assay Babesia microti Antibodies (IgG, IgM) |
| 86666 |
Antibody; Ehrlichia | |
| 86619 |
Antibody; Borrelia (relapsing fever) | |
| 87207 | Smear, primary source with interpretation; special stain for inclusion bodies or parasites (e.g., malaria, coccidia, microsporidia, trypanosomes, herpes viruses | |
| 86750 |
Antibody; Plasmodium (malaria) | |
| 86753 |
Antibody; protozoa, not elsewhere specified | |
| 86788 |
Antibody; West Nile virus, IgM |
|
| 86789 |
Antibody; West Nile virus | |
| 86790 |
Antibody; (Dengue Fever Antibodies; CTF Antibodies),virus, not elsewhere specified | |
| 86794 | Antibody; Zika virus, IgM | |
| 87207 | Smear, primary source with interpretation; special stain for inclusion bodies or parasites (e.g., malaria, coccidia, microsporidia, trypanosomes, herpes viruses | |
| 87449 |
Dengue Virus NS1 Antigen Infectious agent (DENV), antigen detection by immunoassay technique, (e.g., enzyme immunoassay [EIA], enzyme-linked immunosorbent assay [ELISA], immunochemiluminometric assay [IMCA]), qualitative or semiquantitative; multiple-step method, not otherwise specified, each organism |
|
| 87662 | Infectious agent detection by nucleic acid (DNA or RNA); Zika virus, amplified probe technique | |
| 87899 |
Infectious agent detection by nucleic acid (DNA or RNA), not otherwise specified; amplified probe technique, each organism | |
| 0043U |
Tick-borne relapsing fever Borrelia group, antibody detection to 4 recombinant protein groups, by immunoblot, IgM Proprietary test: Tick-Borne Relapsing Fever Borrelia (TBRF) ImmunoBlots IgM Test Lab/Manufacturer: IGeneX Inc |
|
| 0044U |
Tick-borne relapsing fever Borrelia group, antibody detection to 4 recombinant protein groups, by immunoblot, IgG Proprietary test: Tick-Borne Relapsing Fever Borrelia (TBRF) ImmunoBlots IgG Test Lab/Manufacturer: IGeneX Inc |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2018 Forward
| 10/15/2024 | Annual review with major policy revisions, some related to CDC updates of hard vs soft tickborne relapsing fever, Borrelia, Zika and West Nile. Updating notes for clarity and consistency, updating rationale and references. |
| 07/29/2024 | Change review date to 10/01/2024. |
| 08/01/2023 | Annual review, migrating information from CAM 153 Zika Virus Risk Assessment to this policy. Updating title of this policy to "Testing for Vector-Borne Infections". Updating policy, description, notes, rationale and references. Adding CPT 86794 and 87662. Criteria for Zika is being updated to indicate testing for Dengue may be more appropriate than Zika testing due to prevalence. |
| 11/03/2022 | Annual review, policy reformatted for clarity with addition of notes detailing specific symptoms for individual issues. Also adding table of terminology, updating rationale, references and coding. |
| 10/18/2021 |
Annual review, updating policy per updated CDC guidelines for IFA assays and IgG. Also updating description, rationale and references. |
| 10/01/2020 |
Annual review, no change to policy intent. Updating coding and references. |
| 10/22/2019 |
Annual review, revision of status of NAAT and PCR testing in relation to tick borne relapsing fever and babesioisis. NO other changes to policy. |
| 10/02/2019 |
Updating Annual review date. No other changes made |
| 11/26/2018 |
New Policy |